1. Olsson R, Remberger M, Schaffer M, Berggren DM, Svahn BM, Mattsson J, et al. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transplant. 2013; 48(4):537–543. PMID:
23222384.

2. Cluzeau T, Lambert J, Raus N, Dessaux K, Absi L, Delbos F, et al. Risk factors and outcome of graft failure after HLA matched and mismatched unrelated donor hematopoietic stem cell transplantation: a study on behalf of SFGM-TC and SFHI. Bone Marrow Transplant. 2016; 51(5):687–691. PMID:
26855158.

3. Piccin A, McCann S, Socié G, Oneto R, Bacigalupo A, Locasciulli A, et al. Survival of patients with documented autologous recovery after SCT for severe aplastic anemia: a study by the WPSAA of the EBMT. Bone Marrow Transplant. 2010; 45(6):1008–1013. PMID:
19915627.

4. Olsson RF, Logan BR, Chaudhury S, Zhu X, Akpek G, Bolwell BJ, et al. Primary graft failure after myeloablative allogeneic hematopoietic cell transplantation for hematologic malignancies. Leukemia. 2015; 29(8):1754–1762. PMID:
25772027.

5. Rondón G, Saliba RM, Khouri I, Giralt S, Chan K, Jabbour E, et al. Long-term follow-up of patients who experienced graft failure postallogeneic progenitor cell transplantation. Results of a single institution analysis. Biol Blood Marrow Transplant. 2008; 14(8):859–866. PMID:
18640568.

6. Champlin RE, Horowitz MM, van Bekkum DW, Camitta BM, Elfenbein GE, Gale RP, et al. Graft failure following bone marrow transplantation for severe aplastic anemia: risk factors and treatment results. Blood. 1989; 73(2):606–613. PMID:
2644980.

7. Petersdorf EW. Risk assessment in haematopoietic stem cell transplantation: histocompatibility. Best Pract Res Clin Haematol. 2007; 20(2):155–170. PMID:
17448954.

8. Alchalby H, Yunus DR, Zabelina T, Ayuk F, Kröger N. Incidence and risk factors of poor graft function after allogeneic stem cell transplantation for myelofibrosis. Bone Marrow Transplant. 2016; 51(9):1223–1227. PMID:
27088376.

9. Storb R, Prentice RL, Thomas ED. Marrow transplantation for treatment of aplastic anemia. An analysis of factors associated with graft rejection. N Engl J Med. 1977; 296(2):61–66. PMID:
136605.
10. Saad A, Lamb LS. Ex vivo T-cell depletion in allogeneic hematopoietic stem cell transplant: past, present and future. Bone Marrow Transplant. 2017; 52(9):1241–1248. PMID:
28319073.

11. Beatty PG, Clift RA, Mickelson EM, Nisperos BB, Flournoy N, Martin PJ, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985; 313(13):765–771. PMID:
3897863.

12. Remberger M, Watz E, Ringdén O, Mattsson J, Shanwell A, Wikman A. Major ABO blood group mismatch increases the risk for graft failure after unrelated donor hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007; 13(6):675–682. PMID:
17531777.

13. Anasetti C, Amos D, Beatty PG, Appelbaum FR, Bensinger W, Buckner CD, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989; 320(4):197–204. PMID:
2643045.

14. Gaziev J, Sodani P, Lucarelli G, Polchi P, Marktel S, Paciaroni K, et al. Second hematopoietic SCT in patients with thalassemia recurrence following rejection of the first graft. Bone Marrow Transplant. 2008; 42(6):397–404. PMID:
18574445.

15. Massenkeil G, Nagy M, Neuburger S, Tamm I, Lutz C, le Coutre P, et al. Survival after reduced-intensity conditioning is not inferior to standard high-dose conditioning before allogeneic haematopoietic cell transplantation in acute leukaemias. Bone Marrow Transplant. 2005; 36(8):683–689. PMID:
16113673.

16. Brunstein CG, Hirsch BA, Miller JS, McGlennen RC, Verfaillie CM, McGlave PB, et al. Non-leukemic autologous reconstitution after allogeneic bone marrow transplantation for Ph-positive chronic myelogenous leukemia: extended remission preceding eventual relapse. Bone Marrow Transplant. 2000; 26(11):1173–1177. PMID:
11149727.

17. Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007; 40(4):381–387. PMID:
17563735.

18. Klein JP, Moeschberger ML, editors. Survival Analysis: Techniques for Censored and Truncated Data. Berlin, Germany: Springer Science & Business Media;2006.
19. Bradburn MJ, Clark TG, Love SB, Altman DG. Survival analysis part II: multivariate data analysis--an introduction to concepts and methods. Br J Cancer. 2003; 89(3):431–436. PMID:
12888808.
20. Schriber J, Agovi MA, Ho V, Ballen KK, Bacigalupo A, Lazarus HM, et al. Second unrelated donor hematopoietic cell transplantation for primary graft failure. Biol Blood Marrow Transplant. 2010; 16(8):1099–1106. PMID:
20172038.

21. Rubio MT, Savani BN, Labopin M, Polge E, Niederwieser D, Ganser A, et al. The impact of HLA-matching on reduced intensity conditioning regimen unrelated donor allogeneic stem cell transplantation for acute myeloid leukemia in patients above 50 years-a report from the EBMT acute leukemia working party. J Hematol Oncol. 2016; 9(1):65. PMID:
27488518.

22. Harada K, Fuji S, Seo S, Kanda J, Ueki T, Kimura F, et al. Comparison of the outcomes after haploidentical and cord blood salvage transplantations for graft failure following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2020; 55(9):1784–1795. PMID:
32051535.

23. Rudakova T, Kulagin A, Moiseev I, Bykova T, Bondarenko S, Barabanshikova M, et al. Thrombopoietin receptor agonists for treatment of poor graft function after allogeneic hematopoietic stem cell transplantation in adults. Cell Ther Transplant. 2019; 8(2):38–44.

24. Kato M, Nakasone H, Nakano N, Fuji S, Shinohara A, Yokoyama H, et al. Clinical course of autologous recovery with chromosomal abnormalities after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2020; 55(6):1023–1028. PMID:
31819152.

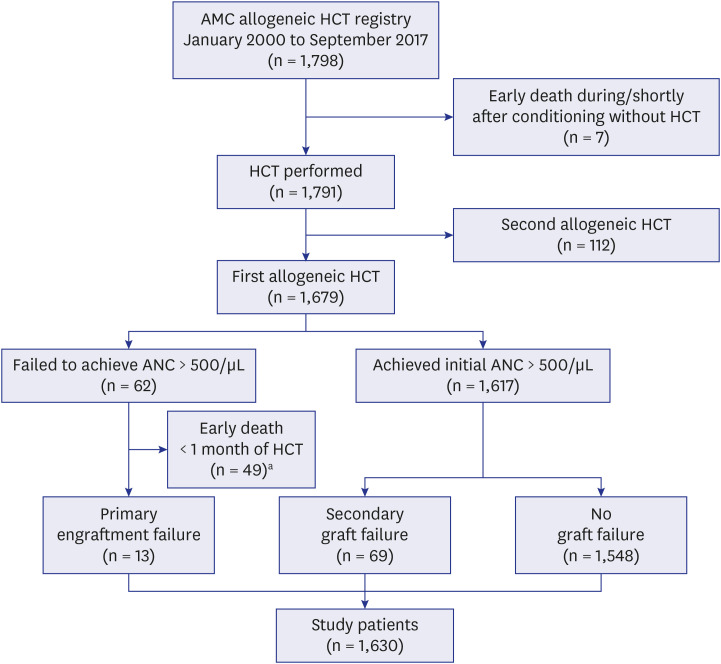
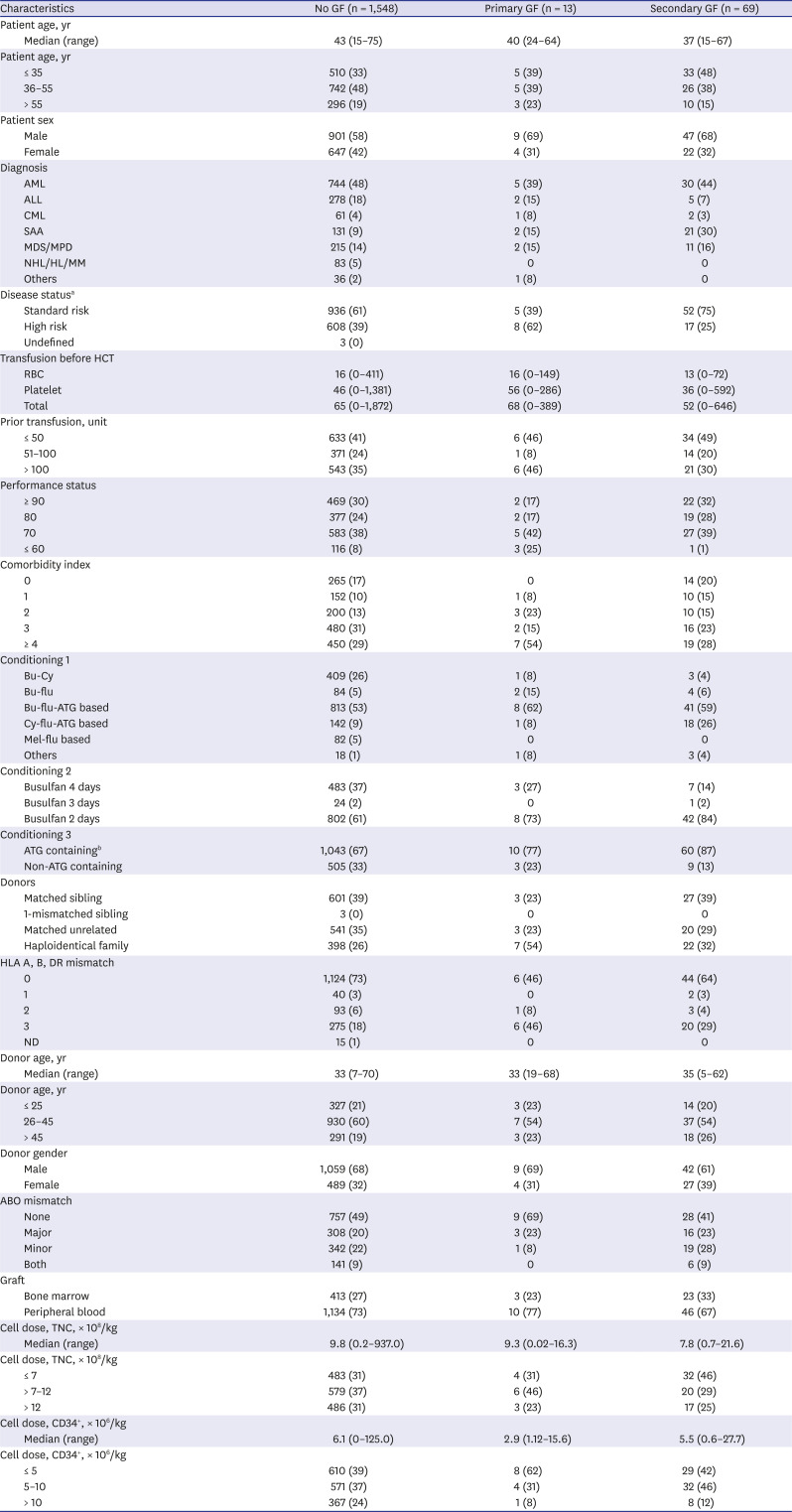
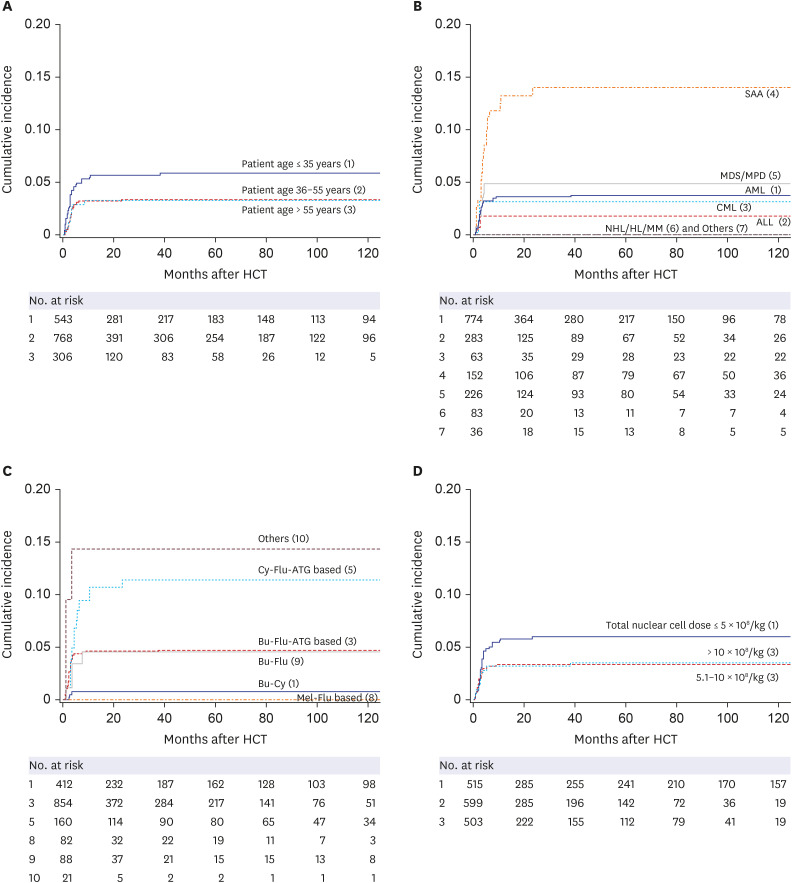
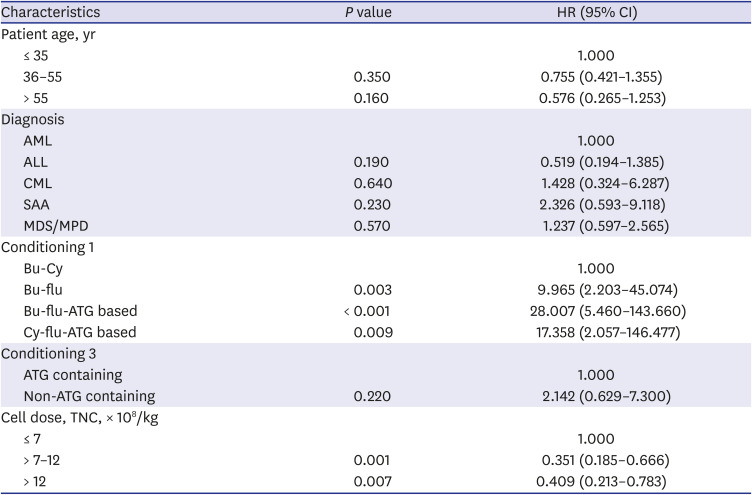




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download