Abstract
We conducted a prospective, mobile-based survey on the self-reported adverse reactions in healthcare workers (HCWs) who received both doses of the BNT162b2 mRNA vaccine. Of the 342 HCWs who completed the two-dose vaccination, 265 (77.5%) responded to the survey at least once. Overall, the rates of adverse reactions were higher after the second dose compared with the first dose (89.1% vs. 80.1%, P = 0.006). The most common systemic reactions were muscle ache (69.1%), fatigue (65.7%), headache (48.7%), chills (44.2%), and fever (32.1%), and were notably more common after the second dose vaccine as well. We also noted a sex difference in which the frequency of adverse reactions after the second dose of the vaccine was significantly higher in females, which was not observed after the first dose. The rates of adverse reactions were lower in older age groups, and the rates and severities of the adverse reactions decreased during the 3-day period following vaccination.
Graphical Abstract
Although the clinical trials of the BNT162b2 mRNA vaccine provided a general overview of the local and systemic reactions following injection in a large number of participants, detailed surveillance of the adverse reactions in different settings is needed to provide further useful information to clinicians and the public.123 As a part of the national coronavirus disease 2019 (COVID-19) vaccination initiative in South Korea, healthcare workers (HCWs) at Asan Medical Center who were at risk of direct contact with COVID-19 patients were administered the BNT162b2 mRNA vaccine between March 11, 2021 and April 3, 2021. We therefore conducted a prospective survey-based study on the HCWs who received the BNT162b2 mRNA vaccine to gather detailed information on the vaccine-associated adverse reactions.
The vaccine was administered according to the manufacturer's instructions as a two-dose regimen with an interval of 3 weeks. All individuals receiving each dose of the vaccine were asked to use a mobile self-report questionnaire form to report any adverse reaction during the 3 days after the injection. We collected information on the sex, age group, number of days after vaccination, use of antipyretic drugs including acetaminophen and nonsteroidal anti-inflammatory drugs, and adverse reactions, which were categorized into 34 local and systemic adverse reactions. The prevalence of adverse reactions was compared according to sex and age groups; moreover, the rates of adverse reactions between the first dose and second dose of the BNT162b2 vaccine were compared by referring to the data from our previous study conducted in the same cohort.4 Categorical variables were compared using Pearson's χ2 test. Trend analysis using the Cochran-Armitage test was performed to identify significant increases or decreases in the frequency of adverse reactions across age groups. P values less than 0.05 were considered statistically significant. Data were analyzed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA) or R version 4.0.4 (R Project for Statistical Computing, Vienna, Austria).
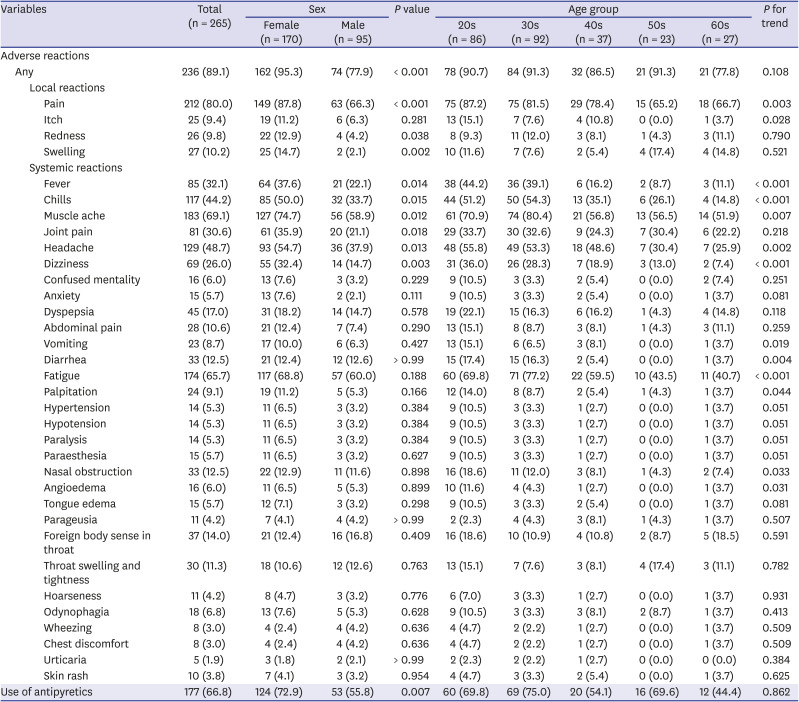
A total of 342 HCWs received the second dose of the BNT162b2 vaccine. Of them, 265 (77.5%) responded at least once to the mobile self-report questionnaires; 81.1% were under 50 years of age and 64.2% were women. Overall, 89.1% experienced at least one adverse reaction of any severity during the first 3 days following the second dose of vaccination (Table 1). Pain at the injection site (80.0%) was the most common local reaction. Of the systemic reactions, muscle ache (69.1%) was the most common form, followed by fatigue (65.7%), headache (48.7%), chills (44.2%), and fever (32.1%). Adverse reactions were more common in females than in males (95% vs. 78%, P < 0.001); significant differences in the prevalence according to sex were noted in local reactions including pain, redness, and swelling, and systemic reactions including fever, chills, muscle ache, joint pain, headache, and dizziness. According to age groups, no significant differences were observed in the frequency of overall adverse reactions (P for trend = 0.108). However, in terms of individual categories, the frequencies of local pain, local itch, fever, chills, muscle ache, headache, dizziness, vomiting, diarrhea, fatigue, palpitation, nasal obstruction, and angioedema showed a significant decreasing trend with age (Table 1). After stratifying by age groups, only those in the 20s and 30s showed significant differences in the overall rates of adverse reactions according to sex; as for individual symptom categories, only the local injection site pain showed significant differences according to sex in the age-stratified analysis (Supplementary Table 2). In the sex-stratified analysis, fewer symptom categories showed significant trends of decreasing frequencies according to age groups (Supplementary Table 3).
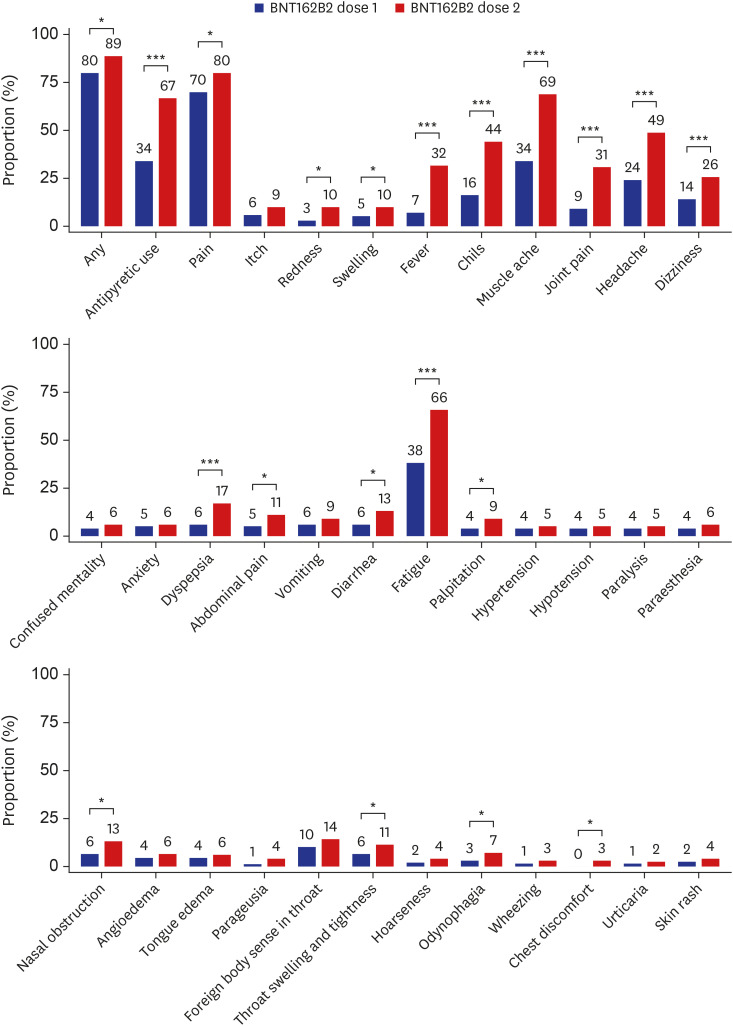
We have recently reported the rates of adverse reactions after the first dose of the BNT162b2 vaccine from the same cohort4; by using the data from the previous report, we compared the rates of adverse reactions between the first and second doses of the BNT162b2 vaccine (Fig. 1). The prevalence of most symptoms was significantly higher after the second dose BNT162b2 vaccine compared with the first dose; particularly, systemic reactions such as fever, chills, muscle ache, joint pain, headache, and fatigue were > 20% more common after the second dose. Similarly, the use of antipyretic or pain medication was significantly more common following the second dose than the first dose (66.8% vs. 34.3%, P < 0.001).
Supplementary Table 1 shows the frequency of the adverse reactions of the two doses of the BNT162b2 vaccine according to their severity over the 3-day reporting period. Similar to the first dose of the vaccine, the frequency of the adverse reactions decreased with time after the second dose of the vaccine. The severities of the systemic adverse reactions including fever, chills, muscle ache, joint pain, headache, and fatigue were higher after the second dose compared with the first dose of the vaccine.
Our previous report4 also delineated the frequencies of adverse reactions after the first dose of the ChAdOx1 nCoV-19 vaccine in the same cohort; as such, we compared the frequencies of adverse reactions between the second dose of the BNT162b2 vaccine and the first dose of the ChAdOx1 nCov-19 vaccine (Supplementary Fig. 1). The frequencies of antipyretics use and most adverse reactions were significantly lower after the second dose of the BNT162b2 vaccine compared with the first dose of the ChAdOx1 nCoV-19 vaccine.
In this prospective survey of adverse reactions after the second dose of the BNT162b2 vaccine, we found that both local and systemic adverse reactions were more frequently reported after the second dose than after the first dose, and that the differences were more pronounced in systemic reactions. These findings are in line with the previous studies reporting more common and severe systemic reactogenicity after the second dose of the BNT162b2 vaccine,23 and are also consistent with a large-scale prospective study from the UK in which systemic adverse reactions occurred more frequently after the second dose than after the first dose of the BNT162b2 vaccine but less frequently than after the first dose of the ChAdOx1 nCoV-19 vaccine.5 Currently available data indicate that the frequency and degree of adverse reactions vary according to different populations, with vaccination-related symptoms being less commonly reported in patients with cancer6 or liver transplant recipients7 compared with healthy controls. We also noted that the frequencies of adverse reactions after the second dose of the vaccine were significantly higher in females, which was not observed in our previous study.4 However, the number of symptom categories showing a significant difference was lower in the age- and sex-stratified analyses, possibly due to the reduced sample size after stratification. Our prospective study results provide further detailed information to the healthcare practitioners and the public regarding the wide range of adverse reactions according to sex, age group, days after injection, and severity.
This study has several limitations. First, given the nature of the self-reporting survey, the frequency of the reported adverse reactions may have been over- or underestimated. In addition, the collected adverse reactions in this study were not medically attended events. Second, as the adverse reactions were collected during the 3-day survey period, symptoms persisting or occurring thereafter could not be analyzed. Third, the proportions of the young age group and females at our hospital were high, which limits the generalization of our results in other demographic settings.
In conclusion, the incidence of local and systemic adverse reactions in HCWs was higher after the second dose of the BNT162b2 vaccine compared with the first dose. The differences in the adverse reaction rates between the two doses were greater in systemic adverse reactions and more pronounced in the female group. The relatively higher frequencies of adverse reactions after the second dose of the BNT162b2 vaccine should be taken into account in the planning of mass immunization using the vaccine.
This study was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2021-0589). Informed consent was waived by the Institutional Review Board because of the observational nature of the study and the fact that the patient identifiers were completely encrypted before analysis.
ACKNOWLEDGMENTS
We thank the healthcare workers of Asan Medical Center for their dedication to patient care during the COVID-19 pandemic and for participating in the current investigation.
Notes
Author Contributions:
Conceptualization: Bae S, Kim SH, Lee JH, Kwon HS.
Data curation: Bae S, Kim M, Kwon S, Joo J, Kwak SH, Kim EO.
Formal analysis: Bae S, Lee YW.
Investigation: Lee YW, Lim SY.
Methodology: Lim SY, Lee JH, Kim TB, Kwon HS, Kim SH.
Software: Kim M, Kwon S, Joo J, Kwak SH, Kim EO.
Validation: Lee YW, Lim SY, Lee JH.
Writing - original draft: Lee YW, Lim SY, Bae S, Jung J.
Writing - review & editing: Bae S, Lim JS, Jung J, Kim TB, Kim SH.
References
1. Chapin-Bardales J, Gee J, Myers T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. JAMA. Forthcoming. 2021; DOI: 10.1001/jama.2021.5374.

2. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020; 383(27):2603–2615. PMID: 33301246.

3. Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020; 383(25):2439–2450. PMID: 33053279.

4. Bae S, Lee YW, Lim SY, Lee JH, Lim JS, Lee S, et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. 2021; 36(17):e115. PMID: 33942579.

5. Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis. Forthcoming. 2021; DOI: 10.1016/S1473-3099(21)00224-3.

6. Monin L, Laing AG, Muñoz-Ruiz M, McKenzie DR, Del Molino Del Barrio I, Alaguthurai T, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. Forthcoming. 2021; DOI: 10.1016/S1470-2045(21)00213-8.

7. Rabinowich L, Grupper A, Baruch R, Ben-Yehoyada M, Halperin T, Turner D, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. Forthcoming. 2021; DOI: 10.1016/j.jhep.2021.04.020.

SUPPLEMENTARY MATERIALS
Supplementary Table 1
Severity of adverse reactions over 3 days after dose 1 and dose 2 of the BNT162b2 vaccine
Supplementary Table 2
Age-stratified local and systemic reactions according to sex in the BNT162b2 group
Supplementary Table 3
Sex-stratified local and systemic reactions according to age groups in the BNT162b2 group
Supplementary Fig. 1
Adverse reactions reported within 3 days following the injection of the BNT162b2 vaccine (dose 1 and 2) and the ChAdOx1 nCoV-19 vaccine (dose 1). The numbers indicate the rates (%) of each adverse reaction. Data on the frequency of adverse reactions following the first dose of the ChAdOx1 nCoV-19 and the BNT162b2 vaccines were adopted from our previous work.4
*P < 0.05.
Fig. 1
Adverse reactions reported within 3 days following dose 1 and dose 2 of the BNT162b2 vaccine. The numbers indicate the rates (%) of each adverse reaction. Data on the frequency of adverse reactions following the first dose of the ChAdOx1 nCoV-19 and the BNT162b2 vaccines were adopted from our previous work.4
*P < 0.05, ***P < 0.001.
Table 1
Local adverse reactions, systemic reactions, and antipyretic use after the second dose according to sex and age groups




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download