METHODS
Study design and participants
Healthcare workers (HCWs) at a tertiary hospital in Seoul were scheduled to receive either a chimpanzee adenovirus-vectored vaccine (ChAdOx1 nCoV-19 [AZD1222], AstraZeneca/Oxford) or an mRNA-based vaccine (BNT162b2, Pfizer/BioNTech) between March 5, 2020 and March 26, 2020. The BNT162b2 vaccine was assigned to high-risk HCWs in direct contact with COVID-19 patients, and the ChAdOx1 vaccine was assigned to those involved in general patient care. Individuals with a history of severe allergic reactions to the components of the COVID-19 vaccine, those who pregnant, and those who refused to be vaccinated for personal reasons were excluded from immunization candidates. Both the ChAdOx1 vaccine and the BNT162b2 vaccine were administered according to the manufacturer's instructions. All individuals receiving the first dose of the vaccines were asked to report any adverse reactions during the 3 days post-vaccination through a mobile self-report questionnaire. Prophylactic use of antipyretics was not recommended, but allowed depending on the personal health conditions.
Data collection and outcomes
We collected information on the employee ID code, sex, age group, vaccine type, number of days after vaccination, use of antipyretic drugs including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs), and adverse reactions through the questionnaire survey. A total of 34 adverse reaction items, including local and systemic reactions, were included in the survey. Local adverse reactions included injection-site pain, redness, swelling, induration, and itch. Systemic adverse reactions included malaise, muscle ache, joint pain, fatigue, headache, dizziness, chills, fever, vomiting, diarrhea, abdominal pain, palpitation, altered mental status, and changes in blood pressure. Allergic reactions such as angioedema, urticaria, wheezing, and skin rash were also included. The severity of adverse reactions was graded according to the following criteria: mild (transient or mild discomfort, no interference with daily activity, and no requirement of medical intervention or therapy), moderate (mild-to-moderate limitation in daily activity and no or minimal requirement of medical intervention or therapy), severe (substantial limitation in daily activity and requirement of medical intervention or therapy), or potentially life-threatening (required assessment in the emergency department or admission to a hospital). The questionnaire sheet used in the survey is shown in the
Supplementary Data 1.
Statistical analysis
Categorical variables were compared using Pearson's χ2 test. The rates of adverse reaction were compared between the ChAdOx1 group and the BNT162b2 group, and the frequency and severity of adverse reactions were compared according to sex and age groups in the two groups. Trend analysis was performed to determine a significant increase or decrease in the frequency of adverse reactions across age groups using the Cochran-Armitage test. P values less than 0.05 were considered statistically significant. The data were analyzed using SPSS v21.0 (IBM Co., Armonk, NY, USA) or R, version 4.0.4 (R Project for Statistical Computing, Vienna, Austria).
Ethics statement
The study protocol was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2021-0589), which waived the requirement for written or verbal consent from the participants based on the observational nature of the study and the fact that the patient identifiers were fully encrypted before analysis.
RESULTS
During the study period, a total of 7,625 HCWs received the first dose of either ChAdOx1 (n = 7,282) or BNT162b2 (n = 343) vaccine. Of them, 5866 HCWs (ChAdOx1, n = 5,589 [95.3%]; BNT162b2, n = 277 [4.7%]) responded at least once to the mobile self-report questionnaires (overall response rate = 76.9%) and were included in the analysis (
Fig. 1). The baseline characteristics and adverse reactions of the ChAdOx1 group and the BNT162b2 group are shown in
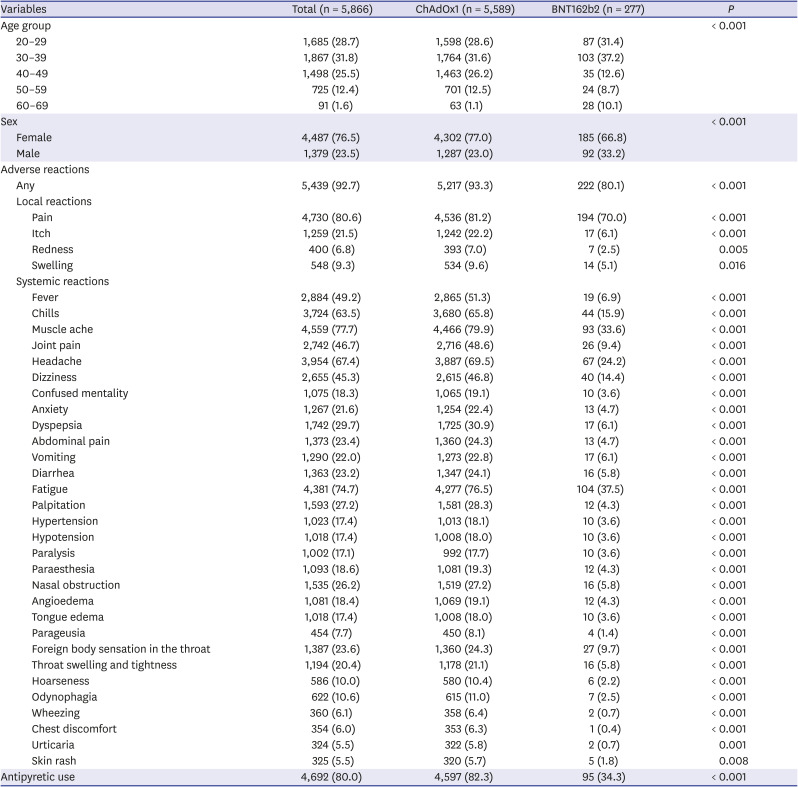
Table 1. Overall, 86.0% of the HCWs were under 50 years of age and 76.5% were women. Of the overall population, 92.7% experienced at least one adverse reaction of any severity during the first 3 days following vaccination, and this rate was significantly higher in the ChAdOx1 group (93.3%) than in the BNT162b2 group (80.1%;
P < 0.001).
Fig. 1
Flow chart of population in this study.
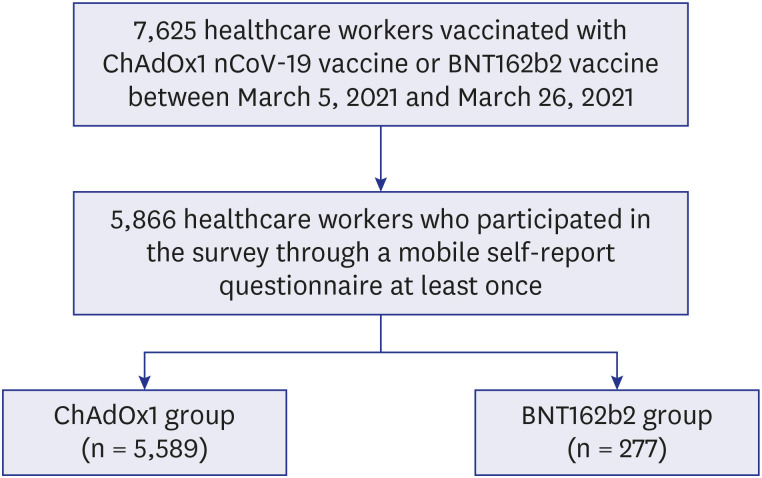
Table 1
Baseline characteristics and adverse reactions according to vaccine type
|
Variables |
Total (n = 5,866) |
ChAdOx1 (n = 5,589) |
BNT162b2 (n = 277) |
P
|
|
Age group |
|
|
|
< 0.001 |
|
20–29 |
1,685 (28.7) |
1,598 (28.6) |
87 (31.4) |
|
30–39 |
1,867 (31.8) |
1,764 (31.6) |
103 (37.2) |
|
40–49 |
1,498 (25.5) |
1,463 (26.2) |
35 (12.6) |
|
50–59 |
725 (12.4) |
701 (12.5) |
24 (8.7) |
|
60–69 |
91 (1.6) |
63 (1.1) |
28 (10.1) |
|
Sex |
|
|
|
< 0.001 |
|
Female |
4,487 (76.5) |
4,302 (77.0) |
185 (66.8) |
|
Male |
1,379 (23.5) |
1,287 (23.0) |
92 (33.2) |
|
Adverse reactions |
|
|
|
|
|
Any |
5,439 (92.7) |
5,217 (93.3) |
222 (80.1) |
< 0.001 |
|
Local reactions |
|
|
|
|
|
|
Pain |
4,730 (80.6) |
4,536 (81.2) |
194 (70.0) |
< 0.001 |
|
|
Itch |
1,259 (21.5) |
1,242 (22.2) |
17 (6.1) |
< 0.001 |
|
|
Redness |
400 (6.8) |
393 (7.0) |
7 (2.5) |
0.005 |
|
|
Swelling |
548 (9.3) |
534 (9.6) |
14 (5.1) |
0.016 |
|
Systemic reactions |
|
|
|
|
|
|
Fever |
2,884 (49.2) |
2,865 (51.3) |
19 (6.9) |
< 0.001 |
|
|
Chills |
3,724 (63.5) |
3,680 (65.8) |
44 (15.9) |
< 0.001 |
|
|
Muscle ache |
4,559 (77.7) |
4,466 (79.9) |
93 (33.6) |
< 0.001 |
|
|
Joint pain |
2,742 (46.7) |
2,716 (48.6) |
26 (9.4) |
< 0.001 |
|
|
Headache |
3,954 (67.4) |
3,887 (69.5) |
67 (24.2) |
< 0.001 |
|
|
Dizziness |
2,655 (45.3) |
2,615 (46.8) |
40 (14.4) |
< 0.001 |
|
|
Confused mentality |
1,075 (18.3) |
1,065 (19.1) |
10 (3.6) |
< 0.001 |
|
|
Anxiety |
1,267 (21.6) |
1,254 (22.4) |
13 (4.7) |
< 0.001 |
|
|
Dyspepsia |
1,742 (29.7) |
1,725 (30.9) |
17 (6.1) |
< 0.001 |
|
|
Abdominal pain |
1,373 (23.4) |
1,360 (24.3) |
13 (4.7) |
< 0.001 |
|
|
Vomiting |
1,290 (22.0) |
1,273 (22.8) |
17 (6.1) |
< 0.001 |
|
|
Diarrhea |
1,363 (23.2) |
1,347 (24.1) |
16 (5.8) |
< 0.001 |
|
|
Fatigue |
4,381 (74.7) |
4,277 (76.5) |
104 (37.5) |
< 0.001 |
|
|
Palpitation |
1,593 (27.2) |
1,581 (28.3) |
12 (4.3) |
< 0.001 |
|
|
Hypertension |
1,023 (17.4) |
1,013 (18.1) |
10 (3.6) |
< 0.001 |
|
|
Hypotension |
1,018 (17.4) |
1,008 (18.0) |
10 (3.6) |
< 0.001 |
|
|
Paralysis |
1,002 (17.1) |
992 (17.7) |
10 (3.6) |
< 0.001 |
|
|
Paraesthesia |
1,093 (18.6) |
1,081 (19.3) |
12 (4.3) |
< 0.001 |
|
|
Nasal obstruction |
1,535 (26.2) |
1,519 (27.2) |
16 (5.8) |
< 0.001 |
|
|
Angioedema |
1,081 (18.4) |
1,069 (19.1) |
12 (4.3) |
< 0.001 |
|
|
Tongue edema |
1,018 (17.4) |
1,008 (18.0) |
10 (3.6) |
< 0.001 |
|
|
Parageusia |
454 (7.7) |
450 (8.1) |
4 (1.4) |
< 0.001 |
|
|
Foreign body sensation in the throat |
1,387 (23.6) |
1,360 (24.3) |
27 (9.7) |
< 0.001 |
|
|
Throat swelling and tightness |
1,194 (20.4) |
1,178 (21.1) |
16 (5.8) |
< 0.001 |
|
|
Hoarseness |
586 (10.0) |
580 (10.4) |
6 (2.2) |
< 0.001 |
|
|
Odynophagia |
622 (10.6) |
615 (11.0) |
7 (2.5) |
< 0.001 |
|
|
Wheezing |
360 (6.1) |
358 (6.4) |
2 (0.7) |
< 0.001 |
|
|
Chest discomfort |
354 (6.0) |
353 (6.3) |
1 (0.4) |
< 0.001 |
|
|
Urticaria |
324 (5.5) |
322 (5.8) |
2 (0.7) |
0.001 |
|
|
Skin rash |
325 (5.5) |
320 (5.7) |
5 (1.8) |
0.008 |
|
Antipyretic use |
4,692 (80.0) |
4,597 (82.3) |
95 (34.3) |
< 0.001 |
In all adverse reaction categories, the ChAdOx1 group had higher reporting rates than did the BNT162b2 group. Pain at the injection site was the most common local reaction. Local pain was reported in 4,536 (81.2%) individuals in the ChAdOx1 group and 197 (70.0%) individuals in the BNT162b2 group (
P < 0.001). Muscle ache (79.9%), fatigue (76.5%), and headache (69.5%) were the most common systemic reactions in the ChAdOx1group. Similarly, fatigue (37.5%), muscle ache (33.6%), and headache (24.2%) were the most common systemic reaction in the BNT162b2 group, albeit with significantly lower frequencies than in the ChAdOx1 group (all
P < 0.001). Systemic reactions whose frequency showed an absolute difference of 30% or greater in the ChAdOx1 group compared with the BNT162b2 group were fever, chills, muscle ache, joint pain, headache, dizziness, and fatigue (
Table 1). In particular, neurologic reactions (e.g., paralysis, paraesthesia) and allergy-like reactions (e.g., foreign body sensation in the throat, swelling in the throat) were significantly more commonly reported in the ChAdOx1 group (all
P < 0.001).
The frequency of adverse reactions gradually decreased over the 3 days of self-reporting period in both groups, with the ChAdOx1 group consistently showing significantly higher reporting rates in all categories regardless of the day of reporting (
Supplementary Table 1). The severity of the adverse reactions was also higher in the ChAdOx1 group. Antipyretics use was more common in the ChAdOx1 group regardless of the day of reporting. Systemic symptoms of moderate or greater severity including a fever of 38 degrees or higher were reported in 20–50% in the ChAdOx1 group. In particular, about half of the ChAdOx1 group reported moderate or severe grade events of chills, muscle ache, headache, and fatigue (
Supplementary Table 1).
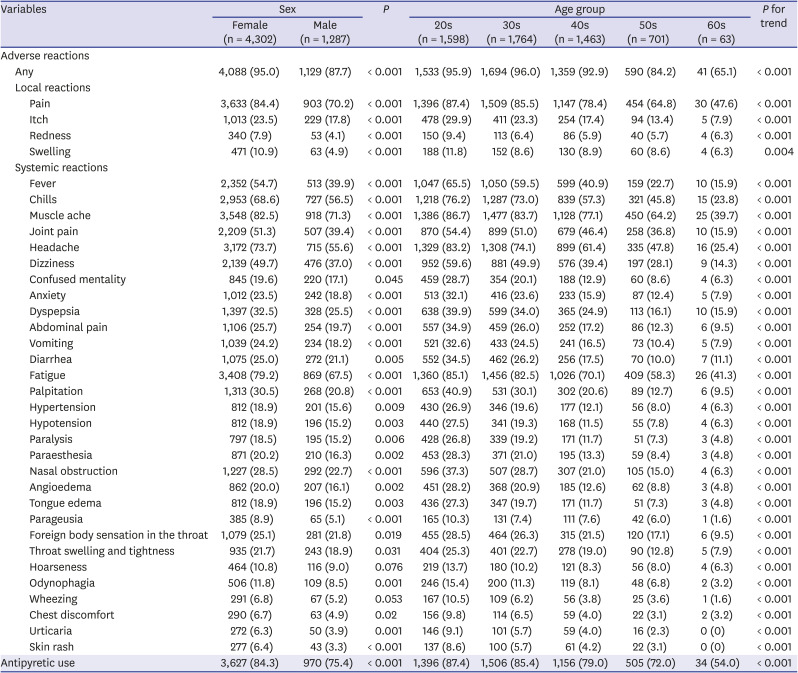
In the ChAdOx1 group, the local and systemic reactions were more frequently reported in females than in males except for hoarseness and wheezing (
Table 2); moreover, all adverse reactions were more significantly more common in the younger age groups (All
P for trend < 0.05). After stratification by age groups, injection site pain was significantly more commonly reported in females in the 20s, 30s, and 40s (
Supplementary Table 2). Rates of systemic reactions including fever, chills, muscle ache, headache, fatigue, and palpitations were higher in females, and the difference according to sex was more evident in the younger age groups. The differences in the frequencies of adverse reactions according to age groups were consistently observed after stratification by sex (
Supplementary Table 3). Most types of adverse reactions in the ChAdOx1 group tended to decrease with older age groups.
Table 2
Local and systemic reactions in the ChAdOx1 group according to sex and age groups
|
Variables |
Sex |
P
|
Age group |
P for trend |
|
Female (n = 4,302) |
Male (n = 1,287) |
20s (n = 1,598) |
30s (n = 1,764) |
40s (n = 1,463) |
50s (n = 701) |
60s (n = 63) |
|
Adverse reactions |
|
|
|
|
|
|
|
|
|
|
Any |
4,088 (95.0) |
1,129 (87.7) |
< 0.001 |
1,533 (95.9) |
1,694 (96.0) |
1,359 (92.9) |
590 (84.2) |
41 (65.1) |
< 0.001 |
|
Local reactions |
|
|
|
|
|
|
|
|
|
|
|
Pain |
3,633 (84.4) |
903 (70.2) |
< 0.001 |
1,396 (87.4) |
1,509 (85.5) |
1,147 (78.4) |
454 (64.8) |
30 (47.6) |
< 0.001 |
|
|
Itch |
1,013 (23.5) |
229 (17.8) |
< 0.001 |
478 (29.9) |
411 (23.3) |
254 (17.4) |
94 (13.4) |
5 (7.9) |
< 0.001 |
|
|
Redness |
340 (7.9) |
53 (4.1) |
< 0.001 |
150 (9.4) |
113 (6.4) |
86 (5.9) |
40 (5.7) |
4 (6.3) |
< 0.001 |
|
|
Swelling |
471 (10.9) |
63 (4.9) |
< 0.001 |
188 (11.8) |
152 (8.6) |
130 (8.9) |
60 (8.6) |
4 (6.3) |
0.004 |
|
Systemic reactions |
|
|
|
|
|
|
|
|
|
|
|
Fever |
2,352 (54.7) |
513 (39.9) |
< 0.001 |
1,047 (65.5) |
1,050 (59.5) |
599 (40.9) |
159 (22.7) |
10 (15.9) |
< 0.001 |
|
|
Chills |
2,953 (68.6) |
727 (56.5) |
< 0.001 |
1,218 (76.2) |
1,287 (73.0) |
839 (57.3) |
321 (45.8) |
15 (23.8) |
< 0.001 |
|
|
Muscle ache |
3,548 (82.5) |
918 (71.3) |
< 0.001 |
1,386 (86.7) |
1,477 (83.7) |
1,128 (77.1) |
450 (64.2) |
25 (39.7) |
< 0.001 |
|
|
Joint pain |
2,209 (51.3) |
507 (39.4) |
< 0.001 |
870 (54.4) |
899 (51.0) |
679 (46.4) |
258 (36.8) |
10 (15.9) |
< 0.001 |
|
|
Headache |
3,172 (73.7) |
715 (55.6) |
< 0.001 |
1,329 (83.2) |
1,308 (74.1) |
899 (61.4) |
335 (47.8) |
16 (25.4) |
< 0.001 |
|
|
Dizziness |
2,139 (49.7) |
476 (37.0) |
< 0.001 |
952 (59.6) |
881 (49.9) |
576 (39.4) |
197 (28.1) |
9 (14.3) |
< 0.001 |
|
|
Confused mentality |
845 (19.6) |
220 (17.1) |
0.045 |
459 (28.7) |
354 (20.1) |
188 (12.9) |
60 (8.6) |
4 (6.3) |
< 0.001 |
|
|
Anxiety |
1,012 (23.5) |
242 (18.8) |
< 0.001 |
513 (32.1) |
416 (23.6) |
233 (15.9) |
87 (12.4) |
5 (7.9) |
< 0.001 |
|
|
Dyspepsia |
1,397 (32.5) |
328 (25.5) |
< 0.001 |
638 (39.9) |
599 (34.0) |
365 (24.9) |
113 (16.1) |
10 (15.9) |
< 0.001 |
|
|
Abdominal pain |
1,106 (25.7) |
254 (19.7) |
< 0.001 |
557 (34.9) |
459 (26.0) |
252 (17.2) |
86 (12.3) |
6 (9.5) |
< 0.001 |
|
|
Vomiting |
1,039 (24.2) |
234 (18.2) |
< 0.001 |
521 (32.6) |
433 (24.5) |
241 (16.5) |
73 (10.4) |
5 (7.9) |
< 0.001 |
|
|
Diarrhea |
1,075 (25.0) |
272 (21.1) |
0.005 |
552 (34.5) |
462 (26.2) |
256 (17.5) |
70 (10.0) |
7 (11.1) |
< 0.001 |
|
|
Fatigue |
3,408 (79.2) |
869 (67.5) |
< 0.001 |
1,360 (85.1) |
1,456 (82.5) |
1,026 (70.1) |
409 (58.3) |
26 (41.3) |
< 0.001 |
|
|
Palpitation |
1,313 (30.5) |
268 (20.8) |
< 0.001 |
653 (40.9) |
531 (30.1) |
302 (20.6) |
89 (12.7) |
6 (9.5) |
< 0.001 |
|
|
Hypertension |
812 (18.9) |
201 (15.6) |
0.009 |
430 (26.9) |
346 (19.6) |
177 (12.1) |
56 (8.0) |
4 (6.3) |
< 0.001 |
|
|
Hypotension |
812 (18.9) |
196 (15.2) |
0.003 |
440 (27.5) |
341 (19.3) |
168 (11.5) |
55 (7.8) |
4 (6.3) |
< 0.001 |
|
|
Paralysis |
797 (18.5) |
195 (15.2) |
0.006 |
428 (26.8) |
339 (19.2) |
171 (11.7) |
51 (7.3) |
3 (4.8) |
< 0.001 |
|
|
Paraesthesia |
871 (20.2) |
210 (16.3) |
0.002 |
453 (28.3) |
371 (21.0) |
195 (13.3) |
59 (8.4) |
3 (4.8) |
< 0.001 |
|
|
Nasal obstruction |
1,227 (28.5) |
292 (22.7) |
< 0.001 |
596 (37.3) |
507 (28.7) |
307 (21.0) |
105 (15.0) |
4 (6.3) |
< 0.001 |
|
|
Angioedema |
862 (20.0) |
207 (16.1) |
0.002 |
451 (28.2) |
368 (20.9) |
185 (12.6) |
62 (8.8) |
3 (4.8) |
< 0.001 |
|
|
Tongue edema |
812 (18.9) |
196 (15.2) |
0.003 |
436 (27.3) |
347 (19.7) |
171 (11.7) |
51 (7.3) |
3 (4.8) |
< 0.001 |
|
|
Parageusia |
385 (8.9) |
65 (5.1) |
< 0.001 |
165 (10.3) |
131 (7.4) |
111 (7.6) |
42 (6.0) |
1 (1.6) |
< 0.001 |
|
|
Foreign body sensation in the throat |
1,079 (25.1) |
281 (21.8) |
0.019 |
455 (28.5) |
464 (26.3) |
315 (21.5) |
120 (17.1) |
6 (9.5) |
< 0.001 |
|
|
Throat swelling and tightness |
935 (21.7) |
243 (18.9) |
0.031 |
404 (25.3) |
401 (22.7) |
278 (19.0) |
90 (12.8) |
5 (7.9) |
< 0.001 |
|
|
Hoarseness |
464 (10.8) |
116 (9.0) |
0.076 |
219 (13.7) |
180 (10.2) |
121 (8.3) |
56 (8.0) |
4 (6.3) |
< 0.001 |
|
|
Odynophagia |
506 (11.8) |
109 (8.5) |
0.001 |
246 (15.4) |
200 (11.3) |
119 (8.1) |
48 (6.8) |
2 (3.2) |
< 0.001 |
|
|
Wheezing |
291 (6.8) |
67 (5.2) |
0.053 |
167 (10.5) |
109 (6.2) |
56 (3.8) |
25 (3.6) |
1 (1.6) |
< 0.001 |
|
|
Chest discomfort |
290 (6.7) |
63 (4.9) |
0.02 |
156 (9.8) |
114 (6.5) |
59 (4.0) |
22 (3.1) |
2 (3.2) |
< 0.001 |
|
|
Urticaria |
272 (6.3) |
50 (3.9) |
0.001 |
146 (9.1) |
101 (5.7) |
59 (4.0) |
16 (2.3) |
0 (0) |
< 0.001 |
|
|
Skin rash |
277 (6.4) |
43 (3.3) |
< 0.001 |
137 (8.6) |
100 (5.7) |
61 (4.2) |
22 (3.1) |
0 (0) |
< 0.001 |
|
Antipyretic use |
3,627 (84.3) |
970 (75.4) |
< 0.001 |
1,396 (87.4) |
1,506 (85.4) |
1,156 (79.0) |
505 (72.0) |
34 (54.0) |
< 0.001 |
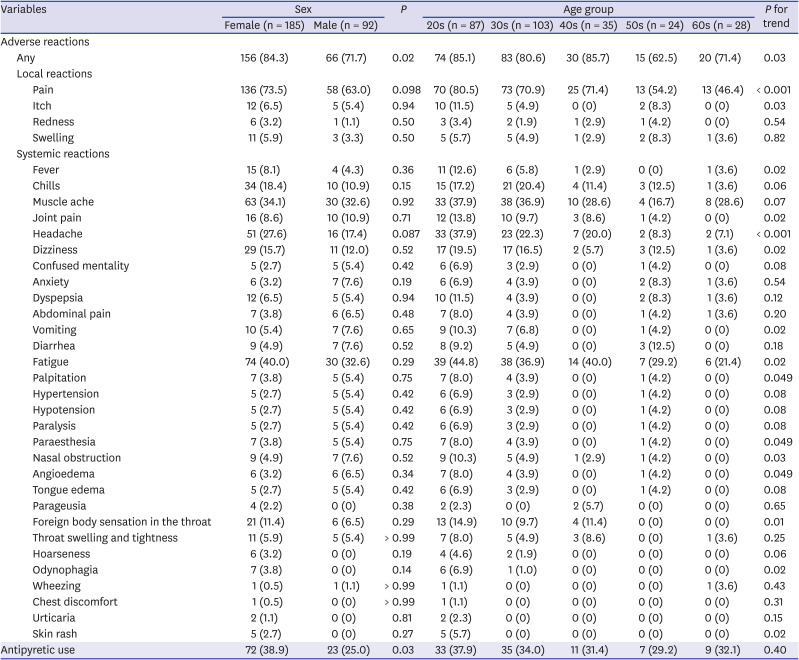
In the BNT162b2 group, there were no significant differences in the frequency of adverse reactions according to sex (
Table 3). The overall rate of adverse reactions was higher in the younger groups (
P for trend= 0.03); however, the number of adverse reactions with significant differences according to age (
Supplementary Table 4) and sex (
Supplementary Table 5) in the stratified analyses were fewer in the BNT162b2 group than in the ChAdOx1 group.
Table 3
Local and systemic reactions in the BNT162b2 group according to sex and age groups
|
Variables |
Sex |
P
|
Age group |
P for trend |
|
Female (n = 185) |
Male (n = 92) |
20s (n = 87) |
30s (n = 103) |
40s (n = 35) |
50s (n = 24) |
60s (n = 28) |
|
Adverse reactions |
|
|
|
|
|
|
|
|
|
|
Any |
156 (84.3) |
66 (71.7) |
0.02 |
74 (85.1) |
83 (80.6) |
30 (85.7) |
15 (62.5) |
20 (71.4) |
0.03 |
|
Local reactions |
|
|
|
|
|
|
|
|
|
|
|
Pain |
136 (73.5) |
58 (63.0) |
0.098 |
70 (80.5) |
73 (70.9) |
25 (71.4) |
13 (54.2) |
13 (46.4) |
< 0.001 |
|
|
Itch |
12 (6.5) |
5 (5.4) |
0.94 |
10 (11.5) |
5 (4.9) |
0 (0) |
2 (8.3) |
0 (0) |
0.03 |
|
|
Redness |
6 (3.2) |
1 (1.1) |
0.50 |
3 (3.4) |
2 (1.9) |
1 (2.9) |
1 (4.2) |
0 (0) |
0.54 |
|
|
Swelling |
11 (5.9) |
3 (3.3) |
0.50 |
5 (5.7) |
5 (4.9) |
1 (2.9) |
2 (8.3) |
1 (3.6) |
0.82 |
|
Systemic reactions |
|
|
|
|
|
|
|
|
|
|
|
Fever |
15 (8.1) |
4 (4.3) |
0.36 |
11 (12.6) |
6 (5.8) |
1 (2.9) |
0 (0) |
1 (3.6) |
0.02 |
|
|
Chills |
34 (18.4) |
10 (10.9) |
0.15 |
15 (17.2) |
21 (20.4) |
4 (11.4) |
3 (12.5) |
1 (3.6) |
0.06 |
|
|
Muscle ache |
63 (34.1) |
30 (32.6) |
0.92 |
33 (37.9) |
38 (36.9) |
10 (28.6) |
4 (16.7) |
8 (28.6) |
0.07 |
|
|
Joint pain |
16 (8.6) |
10 (10.9) |
0.71 |
12 (13.8) |
10 (9.7) |
3 (8.6) |
1 (4.2) |
0 (0) |
0.02 |
|
|
Headache |
51 (27.6) |
16 (17.4) |
0.087 |
33 (37.9) |
23 (22.3) |
7 (20.0) |
2 (8.3) |
2 (7.1) |
< 0.001 |
|
|
Dizziness |
29 (15.7) |
11 (12.0) |
0.52 |
17 (19.5) |
17 (16.5) |
2 (5.7) |
3 (12.5) |
1 (3.6) |
0.02 |
|
|
Confused mentality |
5 (2.7) |
5 (5.4) |
0.42 |
6 (6.9) |
3 (2.9) |
0 (0) |
1 (4.2) |
0 (0) |
0.08 |
|
|
Anxiety |
6 (3.2) |
7 (7.6) |
0.19 |
6 (6.9) |
4 (3.9) |
0 (0) |
2 (8.3) |
1 (3.6) |
0.54 |
|
|
Dyspepsia |
12 (6.5) |
5 (5.4) |
0.94 |
10 (11.5) |
4 (3.9) |
0 (0) |
2 (8.3) |
1 (3.6) |
0.12 |
|
|
Abdominal pain |
7 (3.8) |
6 (6.5) |
0.48 |
7 (8.0) |
4 (3.9) |
0 (0) |
1 (4.2) |
1 (3.6) |
0.20 |
|
|
Vomiting |
10 (5.4) |
7 (7.6) |
0.65 |
9 (10.3) |
7 (6.8) |
0 (0) |
1 (4.2) |
0 (0) |
0.02 |
|
|
Diarrhea |
9 (4.9) |
7 (7.6) |
0.52 |
8 (9.2) |
5 (4.9) |
0 (0) |
3 (12.5) |
0 (0) |
0.18 |
|
|
Fatigue |
74 (40.0) |
30 (32.6) |
0.29 |
39 (44.8) |
38 (36.9) |
14 (40.0) |
7 (29.2) |
6 (21.4) |
0.02 |
|
|
Palpitation |
7 (3.8) |
5 (5.4) |
0.75 |
7 (8.0) |
4 (3.9) |
0 (0) |
1 (4.2) |
0 (0) |
0.049 |
|
|
Hypertension |
5 (2.7) |
5 (5.4) |
0.42 |
6 (6.9) |
3 (2.9) |
0 (0) |
1 (4.2) |
0 (0) |
0.08 |
|
|
Hypotension |
5 (2.7) |
5 (5.4) |
0.42 |
6 (6.9) |
3 (2.9) |
0 (0) |
1 (4.2) |
0 (0) |
0.08 |
|
|
Paralysis |
5 (2.7) |
5 (5.4) |
0.42 |
6 (6.9) |
3 (2.9) |
0 (0) |
1 (4.2) |
0 (0) |
0.08 |
|
|
Paraesthesia |
7 (3.8) |
5 (5.4) |
0.75 |
7 (8.0) |
4 (3.9) |
0 (0) |
1 (4.2) |
0 (0) |
0.049 |
|
|
Nasal obstruction |
9 (4.9) |
7 (7.6) |
0.52 |
9 (10.3) |
5 (4.9) |
1 (2.9) |
1 (4.2) |
0 (0) |
0.03 |
|
|
Angioedema |
6 (3.2) |
6 (6.5) |
0.34 |
7 (8.0) |
4 (3.9) |
0 (0) |
1 (4.2) |
0 (0) |
0.049 |
|
|
Tongue edema |
5 (2.7) |
5 (5.4) |
0.42 |
6 (6.9) |
3 (2.9) |
0 (0) |
1 (4.2) |
0 (0) |
0.08 |
|
|
Parageusia |
4 (2.2) |
0 (0) |
0.38 |
2 (2.3) |
0 (0) |
2 (5.7) |
0 (0) |
0 (0) |
0.65 |
|
|
Foreign body sensation in the throat |
21 (11.4) |
6 (6.5) |
0.29 |
13 (14.9) |
10 (9.7) |
4 (11.4) |
0 (0) |
0 (0) |
0.01 |
|
|
Throat swelling and tightness |
11 (5.9) |
5 (5.4) |
> 0.99 |
7 (8.0) |
5 (4.9) |
3 (8.6) |
0 (0) |
1 (3.6) |
0.25 |
|
|
Hoarseness |
6 (3.2) |
0 (0) |
0.19 |
4 (4.6) |
2 (1.9) |
0 (0) |
0 (0) |
0 (0) |
0.06 |
|
|
Odynophagia |
7 (3.8) |
0 (0) |
0.14 |
6 (6.9) |
1 (1.0) |
0 (0) |
0 (0) |
0 (0) |
0.02 |
|
|
Wheezing |
1 (0.5) |
1 (1.1) |
> 0.99 |
1 (1.1) |
0 (0) |
0 (0) |
0 (0) |
1 (3.6) |
0.43 |
|
|
Chest discomfort |
1 (0.5) |
0 (0) |
> 0.99 |
1 (1.1) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0.31 |
|
|
Urticaria |
2 (1.1) |
0 (0) |
0.81 |
2 (2.3) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0.15 |
|
|
Skin rash |
5 (2.7) |
0 (0) |
0.27 |
5 (5.7) |
0 (0) |
0 (0) |
0 (0) |
0 (0) |
0.02 |
|
Antipyretic use |
72 (38.9) |
23 (25.0) |
0.03 |
33 (37.9) |
35 (34.0) |
11 (31.4) |
7 (29.2) |
9 (32.1) |
0.40 |
DISCUSSION
In this prospective survey of adverse reactions associated with two types of vaccines against COVID-19, the overall adverse reaction rates were 93% and 80% in the ChAdOx1 group and BNT162b2 group, respectively. Between the two types of vaccines, both local and systemic reactions were more commonly reported in the ChAdOx1 group than in the BNT162b2 group. Within the ChAdOx1 group, adverse reactions were significantly more frequent in females and in younger age groups. On the other hand, in the BNT162b2 group, there was no significant difference in the frequency of adverse reactions according to sex, and the number of categories showing a significant trend according to age group was small.
The overall frequency of adverse reactions and the types of commonly reported symptoms in the study group were similar to those reported in the clinical trials of ChAdOx1 and BNT162b2 vaccines. In the clinical trials of the ChAdOx1 vaccine, 61–88% and 65–86% of participants reported local and systemic symptoms following the first dose, respectively.
3 In clinical trials of the BNT162b2 vaccine, 85% and 77% of participants reported at least one local and systemic reaction, respectively, during the 7 day-period after vaccination.
145 However, there are limited data on the comparison of the adverse effects between ChAdOx1 and BNT162b2 in a concurrent cohort. Our study clearly showed that the frequency and severity of systemic reactions were significantly higher in HCWs who received ChAdOx1 compared with those who received BNT162b2 during the same period. The higher frequency and more severe degrees of the adverse reactions in the ChAdOx1 group may be due to the robust innate immune response activated by the adenoviral vector of the ChAdOx1 vaccine.
6 Interestingly, the adverse reactions after the second dose of the ChAdOx1 vaccine were less common than those after its first dose,
3 while the adverse reactions after the second dose of the BNT162b2 vaccine were more common than those after its first dose.
7 Further studies are needed in this area.
It should also be noted that about one-fifth of HCWs receiving ChAdOx1 reported neurologic or allergy-like reactions, such as paralysis, paraesthesia, angioedema, and foreign body sensation in the throat, which were unsolicited adverse reactions in previous clinical trials.
28 Therefore, clinicians should also be aware of the possibility of neurologic or allergy-like reactions in individuals receiving the first dose of the ChAdOx1 vaccine. Upper respiratory symptoms such as foreign body sensation in the throat, throat swelling and tightness, hoarseness, and chest discomfort were significantly more common in the ChAdOx1 group than in the BNT162b2 group. It is not clear whether these upper respiratory symptoms might be due to the abundance of adenoviral receptors in the upper respiratory tract. Initially, these symptoms were confused with the initial symptoms of anaphylaxis, which led to many HCWs being over-treated due to the concern of anaphylaxis. However, these symptoms were quite common and disappeared shortly. Therefore, a more focused approach is needed on the evaluation of anaphylaxis in individuals who show worsening or persistent respiratory symptoms. Furthermore, about 3.5% and 21% of ChAdOx1 group reported high fever (> 39.0°C) and moderate fever (38.0–39.0°C), respectively, while only 0.8% of BNT162b2 reported moderate fever. These data provide useful information on the on-demand use of antipyretics and the necessity of COVID-19 testing according to the prevalence of COVID-19 in the community.
We found that adverse events after the first dose of COVID-19 vaccines were more frequent in female HCWs than in male HCWs. This phenomenon was observed even after adjustment for age. Previous studies also reported that allergic reactions after COVID-19 vaccine and flu vaccine were more common in women than in men.
910 Possible explanations for this phenomenon include the more frequent reporting of side effects in females and some unknown immunologic difference between the two sexes.
11 It is also worthwhile to note that the occurrence of adverse events after the first dose of ChAdOx1 was less common in older age groups. Considering that a high frequency of systemic adverse events in the elderly might pose a significant barrier in vaccination, our observation provides a rationale for implementing a widespread vaccination program in the elderly population in South Korea.
There are several limitations to this study. First, the frequency and severity of the self-reported adverse reactions may have been biased considering that the HCWs were not blinded to the type of vaccine. Second, the accuracy of the frequency and severity of adverse reactions may have been overestimated or underestimated due to the nature of the self-reporting survey. Also, the reported adverse reactions in this study were not medically-attended adverse events. Third, since the survey was conducted for three days following vaccination, adverse reactions that occurred thereafter were not evaluated.
In conclusion, the frequency and severity of adverse reactions, particularly the systemic reactions, were significantly higher in those who received the ChAdOx1 vaccine than did those who received the BNT162b2 vaccine. The adverse reactions were more commonly reported in females and those in the younger age groups. The frequency and severity of adverse reactions associated with the ChAdOx1 vaccine should be taken into account when planning mass immunization, especially in females and younger age groups.







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download