This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Vaccination against coronavirus disease 2019 (COVID-19) is underway globally to prevent the infection caused by the severe acute respiratory syndrome coronavirus 2. We aimed to investigate the adverse events following immunization (AEFIs) for COVID-19 among healthcare workers (HCWs).
Methods
This was a retrospective study of the AEFIs associated with the first dose of the ChAdOx1 nCoV-19 vaccine at the Kosin University Gospel Hospital from March 3 to March 22, 2021. We investigated the systemic and local adverse events during the 7 days following the vaccination using the Mobile Vaccine Adverse Events Reporting System (MVAERS) developed by our hospital.
Results
A total of 1,503 HCWs were vaccinated, and the data of 994 HCWs were reported in the MVAERS. The most commonly reported AEFIs were tenderness at the injection site (94.5%), fatigue (92.9%), pain at the injection site (88.0%), and malaise (83.8%). The severity of most AEFIs was mild-to-moderate, and the severity and number of AEFIs were less in the older age group. There were no serious events requiring hospitalization, and most AEFIs improved within a few days.
Conclusion
The AEFIs associated with the ChAdOx1 nCoV-19 vaccine were tolerable, and the use of the MVAERS was helpful in monitoring the AEFIs. The use of MVAERS will help in sharing accurate and ample information about vaccination against COVID-19.
Keywords: COVID-19, Vaccination, Adverse Events Following Immunization, AEFI
INTRODUCTION
As of March 2021, coronavirus disease 2019 (COVID-19) has been reported in more than 120 million patients, and there have been more than 2 million deaths worldwide.
1 Currently, the strategy of vaccination against COVID-19 is being implemented to overcome this global catastrophe.
In the Republic of Korea, the COVID-19 vaccination was initiated in February 2021. The priority population vaccinated was the frontline healthcare workers (HCWs). In the first quarter of the vaccination, HCWs were administered the BNT162b2 mRNA vaccine (Pfizer/BioNTech) or ChAdOx1 nCoV-19 vaccine (AstraZeneca). Randomized controlled trials have reported an acceptable safety profile for both vaccines.
23 It is important to improve the inoculation rate of the COVID-19 vaccine to achieve the national goal of herd immunity. However, several adverse events associated with COVID-19 vaccines have been reported, including anaphylaxis, transverse myelitis, and deep vein thrombosis.
345 Disinformation through the mass media has been the cause of considerable anxiety among people about the safety of the vaccine.
6 Moreover, there have been no large-scale research studies about the adverse event following immunization (AEFI) associated with the COVID-19 vaccine in the domestic population.
We aimed to evaluate the incidence and severity of AEFI associated with the COVID-19 vaccine at a single center during the first quarter of vaccination and to provide a basis to ensure safety during the future national vaccination against COVID-19. In this study, we used the medical records of AEFI among HCWs that were voluntarily reported in the Mobile Vaccine Adverse Events Reporting System (MVAERS) developed by our hospital.
METHODS
Study design and population
A retrospective, single-center cohort study was conducted at the Kosin University Gospel Hospital in the Republic of Korea. The study subjects were HCWs who had completed the first dose of the ChAdOx1 nCov-19 vaccine. According to the government policy, only HCWs under the age of 65 years were included. Among those who received the vaccination, HCWs who did not report to the mobile reporting system were excluded.
Vaccination protocol
Information about the ChAdOx1 nCov-19 vaccine and vaccination was notified via intranet e-mail to all HCWs who were recommended for vaccination, and informed consent was obtained one week before the start date of the vaccination. For those who consented to undergo vaccination, the vaccination schedules were set from March 9 to March 16, 2021. The vaccination was conducted by dividing the space of the main auditorium so that preliminary medical examinations, vaccinations, and monitoring could be performed continuously. The HCWs were asked to fill a preliminary form that captured the previous history of vaccination, COVID-19, and allergies. The vaccine was administered in the deltoid region by well-trained nurses, and adverse events were monitored for 15–30 minutes. Every HCW who had a history of allergic reaction was monitored for at least 30 minutes. After monitoring, vaccinated HCWs were advised to report to the outpatient clinic of the department of infectious diseases (IDs) or the emergency room (ER) if the adverse events persisted.
Adverse events reporting system
The MVAERS project developed a mobile web page to systematically capture spontaneous reporting of AEFIs. This web page was used to collect the data of AEFIs that are analyzed in this study. All HCWs received webpage hyperlinks by a short message system twice a day for 7 days from the day of vaccination. Infectious disease physicians monitored the reported adverse events daily.
The AEFI surveillance surveys comprised questions about 12 adverse events and a provision of free-text reporting for any other adverse events. Solicited local AEFIs included tenderness, pain at rest, redness, and swelling at the injection site. Solicited systemic AEFIs included fatigue, headache, malaise, arthralgia, chills, fever, nausea or vomiting, and diarrhea. The severity of the local and systemic AEFIs was graded on a scale of 1 to 4 based on the guidelines of the Korean Ministry of Food and Drug Safety and the U.S. Food and Drug Administration (
Supplementary Table 1).
7 Free-text reporting allowed those vaccinated to describe any other symptoms and record the type and amount of pain medications used. In the case of the HCWs visiting the outpatient clinics or ERs due to AEFIs, the adverse event was reported to the national COVID-19 vaccination management system according to the government's policy.
Statistical analysis
Categorical variables were compared using Pearson's χ2 test. Statistical significance was set at P < 0.05. Statistical analyses were performed using SPSS software (version 25.0; IBM Corp., Armonk, NY, USA).
Ethics statement
This study was reviewed and approved by the Institutional Review Board of Kosin University College of Medicine (approval No. 2021-03-029). Informed consent was waived because of the retrospective nature of the study.
RESULTS
A total of 1,503 HCWs were vaccinated, and the data of 994 HCWs was reported in the MVAERS (994/1,503, 66.1%). The mean age of the respondents was 35.7 years (range, 19–63), and 76.7% were female (762/994). The incidence of AEFIs is shown in
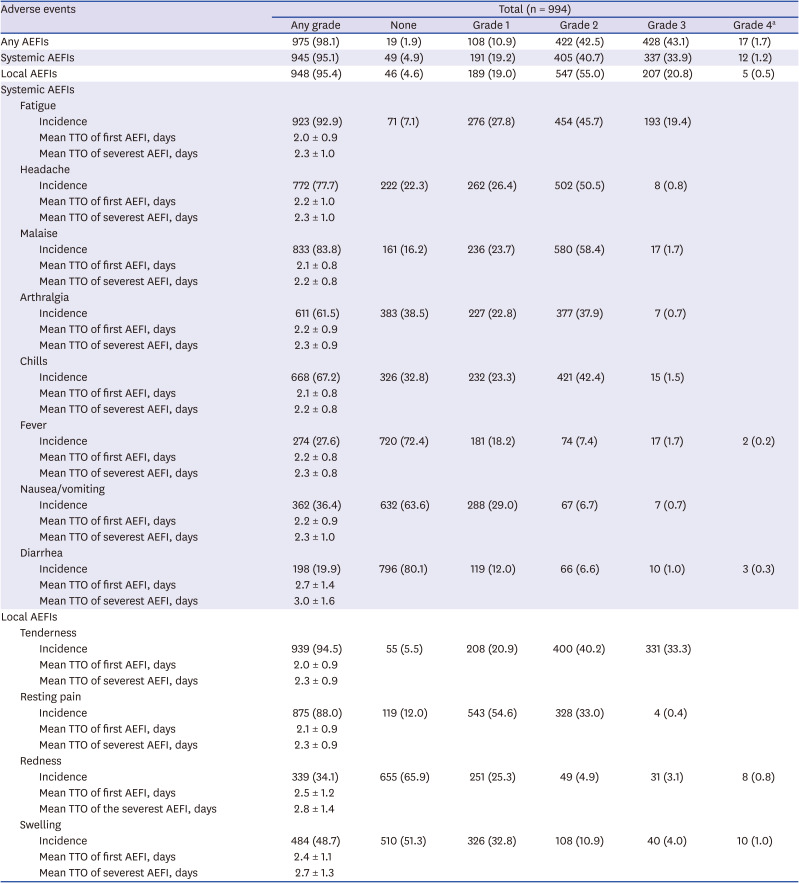
Table 1. Among the respondents, 975 (98.1%) reported more than one AEFI, and the incidence of local AEFIs was similar to that of systemic AEFIs (95.4% and 95.1%, respectively).
Table 1
Incidence, time to onset of the first adverse event and severest episode of AEFI
|
Adverse events |
Total (n = 994) |
|
Any grade |
None |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4a
|
|
Any AEFIs |
975 (98.1) |
19 (1.9) |
108 (10.9) |
422 (42.5) |
428 (43.1) |
17 (1.7) |
|
Systemic AEFIs |
945 (95.1) |
49 (4.9) |
191 (19.2) |
405 (40.7) |
337 (33.9) |
12 (1.2) |
|
Local AEFIs |
948 (95.4) |
46 (4.6) |
189 (19.0) |
547 (55.0) |
207 (20.8) |
5 (0.5) |
|
Systemic AEFIs |
|
|
|
|
|
|
|
Fatigue |
|
|
|
|
|
|
|
|
Incidence |
923 (92.9) |
71 (7.1) |
276 (27.8) |
454 (45.7) |
193 (19.4) |
|
|
|
Mean TTO of first AEFI, days |
2.0 ± 0.9 |
|
|
|
|
|
|
|
Mean TTO of severest AEFI, days |
2.3 ± 1.0 |
|
|
|
|
|
|
Headache |
|
|
|
|
|
|
|
|
Incidence |
772 (77.7) |
222 (22.3) |
262 (26.4) |
502 (50.5) |
8 (0.8) |
|
|
|
Mean TTO of first AEFI, days |
2.2 ± 1.0 |
|
|
|
|
|
|
|
Mean TTO of severest AEFI, days |
2.3 ± 1.0 |
|
|
|
|
|
|
Malaise |
|
|
|
|
|
|
|
|
Incidence |
833 (83.8) |
161 (16.2) |
236 (23.7) |
580 (58.4) |
17 (1.7) |
|
|
|
Mean TTO of first AEFI, days |
2.1 ± 0.8 |
|
|
|
|
|
|
|
Mean TTO of severest AEFI, days |
2.2 ± 0.8 |
|
|
|
|
|
|
Arthralgia |
|
|
|
|
|
|
|
|
Incidence |
611 (61.5) |
383 (38.5) |
227 (22.8) |
377 (37.9) |
7 (0.7) |
|
|
|
Mean TTO of first AEFI, days |
2.2 ± 0.9 |
|
|
|
|
|
|
|
Mean TTO of severest AEFI, days |
2.3 ± 0.9 |
|
|
|
|
|
|
Chills |
|
|
|
|
|
|
|
|
Incidence |
668 (67.2) |
326 (32.8) |
232 (23.3) |
421 (42.4) |
15 (1.5) |
|
|
|
Mean TTO of first AEFI, days |
2.1 ± 0.8 |
|
|
|
|
|
|
|
Mean TTO of severest AEFI, days |
2.2 ± 0.8 |
|
|
|
|
|
|
Fever |
|
|
|
|
|
|
|
|
Incidence |
274 (27.6) |
720 (72.4) |
181 (18.2) |
74 (7.4) |
17 (1.7) |
2 (0.2) |
|
|
Mean TTO of first AEFI, days |
2.2 ± 0.8 |
|
|
|
|
|
|
|
Mean TTO of severest AEFI, days |
2.3 ± 0.8 |
|
|
|
|
|
|
Nausea/vomiting |
|
|
|
|
|
|
|
|
Incidence |
362 (36.4) |
632 (63.6) |
288 (29.0) |
67 (6.7) |
7 (0.7) |
|
|
|
Mean TTO of first AEFI, days |
2.2 ± 0.9 |
|
|
|
|
|
|
|
Mean TTO of severest AEFI, days |
2.3 ± 1.0 |
|
|
|
|
|
|
Diarrhea |
|
|
|
|
|
|
|
|
Incidence |
198 (19.9) |
796 (80.1) |
119 (12.0) |
66 (6.6) |
10 (1.0) |
3 (0.3) |
|
|
Mean TTO of first AEFI, days |
2.7 ± 1.4 |
|
|
|
|
|
|
|
Mean TTO of severest AEFI, days |
3.0 ± 1.6 |
|
|
|
|
|
|
Local AEFIs |
|
|
|
|
|
|
|
Tenderness |
|
|
|
|
|
|
|
|
Incidence |
939 (94.5) |
55 (5.5) |
208 (20.9) |
400 (40.2) |
331 (33.3) |
|
|
|
Mean TTO of first AEFI, days |
2.0 ± 0.9 |
|
|
|
|
|
|
|
Mean TTO of severest AEFI, days |
2.3 ± 0.9 |
|
|
|
|
|
|
Resting pain |
|
|
|
|
|
|
|
|
Incidence |
875 (88.0) |
119 (12.0) |
543 (54.6) |
328 (33.0) |
4 (0.4) |
|
|
|
Mean TTO of first AEFI, days |
2.1 ± 0.9 |
|
|
|
|
|
|
|
Mean TTO of severest AEFI, days |
2.3 ± 0.9 |
|
|
|
|
|
|
Redness |
|
|
|
|
|
|
|
|
Incidence |
339 (34.1) |
655 (65.9) |
251 (25.3) |
49 (4.9) |
31 (3.1) |
8 (0.8) |
|
|
Mean TTO of first AEFI, days |
2.5 ± 1.2 |
|
|
|
|
|
|
|
Mean TTO of the severest AEFI, days |
2.8 ± 1.4 |
|
|
|
|
|
|
Swelling |
|
|
|
|
|
|
|
|
Incidence |
484 (48.7) |
510 (51.3) |
326 (32.8) |
108 (10.9) |
40 (4.0) |
10 (1.0) |
|
|
Mean TTO of first AEFI, days |
2.4 ± 1.1 |
|
|
|
|
|
|
|
Mean TTO of severest AEFI, days |
2.7 ± 1.3 |
|
|
|
|
|

The most commonly reported systemic AEFIs were fatigue (923, 92.9%) and malaise (833, 83.8%). A noticeably low percentage (27.6%) of HCWs reported fever (temperature ≥ 38°C). Two HCWs reported high fever above 40°C (0.2%), and three HCWs reported severe diarrhea (0.3%). Among respondents, grade 1 or 2 pain (871, 87.6%) and tenderness (608, 61.2%) at the injection site were the most commonly reported local AEFIs. Most of the local AEFIs were grade 1 and 2 in severity; however, the frequency of local AEFIs of grade 3 or above was higher than that of systemic AEFIs. The median time from the vaccination to the onset of the first event and the most severe adverse event was similar for all AEFIs (
Table 1). Most AEFIs were observed within the first 1 to 3 days after vaccination and resolved shortly thereafter.
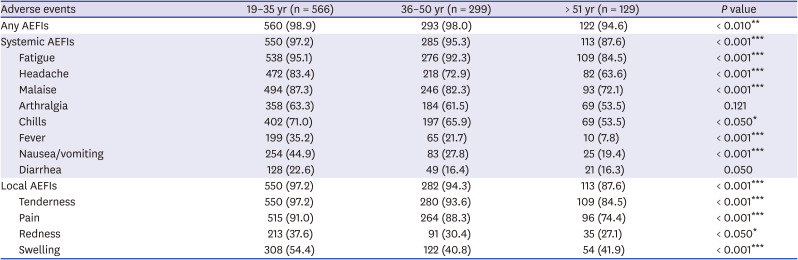
We summarize the incidence of AEFIs by age group in
Table 2. Systemic and local AEFIs were reported more frequently among respondents below 35 years of age (560/566, 98.9%) than among those more than 51 years of age (122/129, 94.6%). In addition, all AEFIs were reported to be more severe in younger HCWs (under 35 years of age) than in older HCWs (
Supplementary Table 2). The incidence and severity of local and systemic AEFIs were significantly higher in the younger HCWs group than in the older HCWs group (
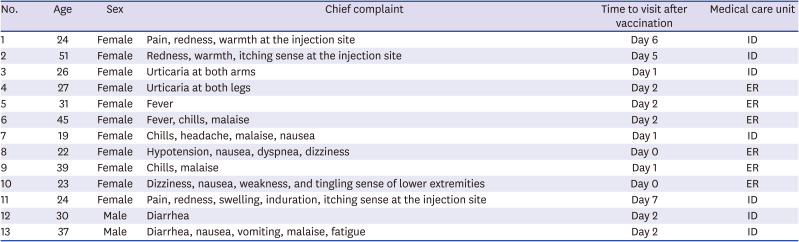
P < 0.05), except for arthralgia and diarrhea. Skin allergic reaction was not included in the survey questions, and fifteen HCWs reported skin allergic reaction such as urticaria and itching at the injection site with allowed free-text. Among the respondents, 13 visited the ER or outpatient clinic at the IDs (
Table 3). Their chief complaints included urticaria, pain at the injection site, and itching, fever, chills, malaise, headache, dizziness, diarrhea, tingling sensation, and weakness of the lower extremities. One patient complained of dyspnea, nausea, and hypotension within 10 minutes after vaccination, and was immediately referred to the ER. Subsequently, the patient improved naturally and was discharged after supportive treatment. No hospitalization was required in any of the cases.
Table 2
Incidence of the adverse events by age group
|
Adverse events |
19–35 yr (n = 566) |
36–50 yr (n = 299) |
> 51 yr (n = 129) |
P value |
|
Any AEFIs |
560 (98.9) |
293 (98.0) |
122 (94.6) |
< 0.010**
|
|
Systemic AEFIs |
550 (97.2) |
285 (95.3) |
113 (87.6) |
< 0.001***
|
|
Fatigue |
538 (95.1) |
276 (92.3) |
109 (84.5) |
< 0.001***
|
|
Headache |
472 (83.4) |
218 (72.9) |
82 (63.6) |
< 0.001***
|
|
Malaise |
494 (87.3) |
246 (82.3) |
93 (72.1) |
< 0.001***
|
|
Arthralgia |
358 (63.3) |
184 (61.5) |
69 (53.5) |
0.121 |
|
Chills |
402 (71.0) |
197 (65.9) |
69 (53.5) |
< 0.050*
|
|
Fever |
199 (35.2) |
65 (21.7) |
10 (7.8) |
< 0.001***
|
|
Nausea/vomiting |
254 (44.9) |
83 (27.8) |
25 (19.4) |
< 0.001***
|
|
Diarrhea |
128 (22.6) |
49 (16.4) |
21 (16.3) |
0.050 |
|
Local AEFIs |
550 (97.2) |
282 (94.3) |
113 (87.6) |
< 0.001***
|
|
Tenderness |
550 (97.2) |
280 (93.6) |
109 (84.5) |
< 0.001***
|
|
Pain |
515 (91.0) |
264 (88.3) |
96 (74.4) |
< 0.001***
|
|
Redness |
213 (37.6) |
91 (30.4) |
35 (27.1) |
< 0.050*
|
|
Swelling |
308 (54.4) |
122 (40.8) |
54 (41.9) |
< 0.001***
|

Table 3
Patients who visited the outpatient clinic or ER due to adverse events following immunization
|
No. |
Age |
Sex |
Chief complaint |
Time to visit after vaccination |
Medical care unit |
|
1 |
24 |
Female |
Pain, redness, warmth at the injection site |
Day 6 |
ID |
|
2 |
51 |
Female |
Redness, warmth, itching sense at the injection site |
Day 5 |
ID |
|
3 |
26 |
Female |
Urticaria at both arms |
Day 1 |
ID |
|
4 |
27 |
Female |
Urticaria at both legs |
Day 2 |
ER |
|
5 |
31 |
Female |
Fever |
Day 2 |
ER |
|
6 |
45 |
Female |
Fever, chills, malaise |
Day 2 |
ER |
|
7 |
19 |
Female |
Chills, headache, malaise, nausea |
Day 1 |
ID |
|
8 |
22 |
Female |
Hypotension, nausea, dyspnea, dizziness |
Day 0 |
ER |
|
9 |
39 |
Female |
Chills, malaise |
Day 1 |
ER |
|
10 |
23 |
Female |
Dizziness, nausea, weakness, and tingling sense of lower extremities |
Day 0 |
ER |
|
11 |
24 |
Female |
Pain, redness, swelling, induration, itching sense at the injection site |
Day 7 |
ID |
|
12 |
30 |
Male |
Diarrhea |
Day 2 |
ID |
|
13 |
37 |
Male |
Diarrhea, nausea, vomiting, malaise, fatigue |
Day 2 |
ID |

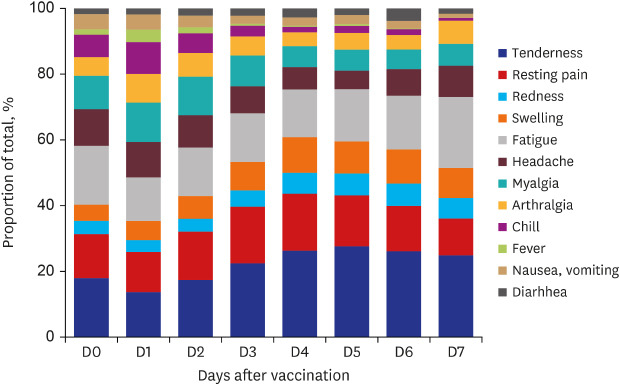
The distribution of the types of AEFIs during the monitoring period is presented in
Fig. 1. On day 1 after vaccination, most AEFIs were reported, and systemic AEFIs tended to improve faster than local AEFIs.
Fig. 1
Distribution of types of adverse events following immunization during monitored period after vaccination.
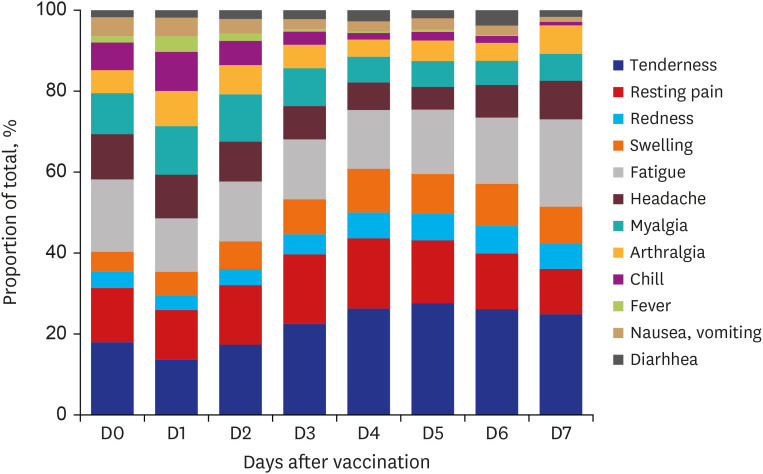

The proportion of respondents who took acetaminophen or nonsteroidal anti-inflammatory drugs for symptomatic relief was 26.4% on day 0 and 47.4% on day 1 after vaccination, and there was a decreasing trend thereafter.
DISCUSSION
We found that the most commonly reported AEFIs after the first dose of the ChAdOx1 nCoV-19 vaccine were fatigue, malaise, tenderness, and pain at rest at the injection site. The severity of most AEFIs was mild-to-moderate, and the severity and number of AEFIs were less in the older age group. There were no serious events requiring hospitalization, and most AEFIs improved within a few days.
In an interim analysis of four clinical trials on the ChAdOx1 nCoV-19 vaccine, the most frequently reported adverse reactions were tenderness at the injection site (63.7%), pain at the injection site (54.2%), headache (52.6%), and fatigue (53.1%). The majority of the adverse reactions were mild-to-moderate in severity and usually resolved within a few days of vaccination.
38 Compared to this report, a higher incidence and severity of local and systemic AEFIs were reported in our study. The proportion of East Asian participants in the previous clinical trials was very low (4.3%, COV001; 5.3%, COV002; 2.5%, COV003; not recorded in COV004); thus, the ethnic differences might be the cause for these differences. In a safety analysis of the clinical trials, 8.9% of the participants were aged 65 or above; in contrast, there were none from this age group in our study. The incidence and severity might have been underestimated due to the involvement of more elderly participants in the clinical trials since these studies reported that reactogenicity was generally milder and reported less frequently in older adults (≥ 65 years old).
Huh et al.
9 reported that the incidence of anaphylaxis associated with vaccination tended to increase in Korea. As of March 26, 2021, according to the status of reports of adverse reactions after vaccination against COVID-19 in Korea, 96 suspected cases of anaphylaxis were reported (96/771,284, 0.01%).
10 However, in this study, only one HCW presented with an acute allergic reaction with hypotension, which spontaneously resolved. Moreover, no serious AEFIs that required hospitalization or death was reported during the monitoring period. These results are consistent with the results of the ChAdOx1 nCoV-19 vaccination among HCWs in Nepal.
11 Such mild-to-moderate AEFIs are acceptable during immunization against COVID-19. These results are helpful in addressing the vaccine hesitancy caused by concerns about severe adverse events associated with the COVID-19 vaccine.
To manage with AEFIs, we developed and used MVAERS during the first quarter of vaccination. The MVAERS made it easy to collect medical records about AEFIs and allowed the infectious disease specialists to monitor the progress of all respondents. It is important to institute a systematic surveillance protocol for safety as much as for the effectiveness of vaccines. By setting up a surveillance program such as a mobile web-based, self-reporting adverse event monitoring system at the institution, adequate management of adverse events is feasible, and it will alleviate the concerns about adverse events. This will also contribute to improving the rate of inoculation.
Our study has several limitations. First, medical records were voluntarily reported by HCWs; thus, they were not objective. Second, since this study was conducted at a single center and comprised subjects below 65 years of age, the results might not be generalizable. Third, we did not capture the comorbidities. The possibility of bias due to unobserved variables cannot be excluded. Fourth, allergic reactions such as urticaria or itching at the injection site were not included in the AEFI surveillance survey.
In conclusion, fatigue and mild-to-moderate pain or tenderness at the injection site were frequently reported after the ChAdOx1 nCoV-19 vaccination. There were no serious events that required hospitalization. To develop a novel vaccination strategy against emerging infectious diseases, the sharing of accurate and abundant information is important, and it will be helpful to use a mobile web-based adverse event monitoring system.
ACKNOWLEDGMENTS
We are grateful to Sung-Lim Song, director of the computer system of Kosin University Gospel Hospital, for his technical assistance in developing an exclusive mobile vaccine adverse event reporting system.
References
1. World Health Organization. WHO coronavirus (COVID-19) dashboard. Updated 2021. Accessed March 23, 2021.
https://covid19.who.int.
2. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020; 383(27):2603–2615. PMID:
33301246.

3. Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021; 397(10269):99–111. PMID:
33306989.
4. CDC COVID-19 Response Team. Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine - United States, December 14–23, 2020. MMWR Morb Mortal Wkly Rep. 2021; 70(2):46–51. PMID:
33444297.
5. Carli G, Nichele I, Ruggeri M, Barra S, Tosetto A. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern Emerg Med. Forthcoming. 2021.

6. Farooq F, Rathore FA. COVID-19 vaccination and the challenge of infodemic and disinformation. J Korean Med Sci. 2021; 36(10):e78. PMID:
33724740.

9. Huh K, Kim YE, Radnaabaatar M, Lee DH, Kim DW, Shin SA, et al. Estimating baseline incidence of conditions potentially associated with vaccine adverse events: a call for surveillance system using the Korean National Health Insurance Claims Data. J Korean Med Sci. 2021; 36(9):e67. PMID:
33686812.

11. Sah R, Shrestha S, Mehta R, Sah SK, Rabaan AA, Dhama K, et al. AZD1222 (Covishield) vaccination for COVID-19: experiences, challenges, and solutions in Nepal. Travel Med Infect Dis. 2021; 40:101989. PMID:
33578045.








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download