INTRODUCTION
MATERIALS AND METHODS
Reagents and cell culture
Cell viability assay
Cell cycle analysis
Annexin V-phycoerythrin (V-PE) binding assay
Western blot analysis
Measurement of ROS and mitochondrial membrane potential
Spheroid culture and viability assay
Spheroid staining
Statistical analysis
RESULTS
The combination of resveratrol and DTX causes synergistic cytotoxicity
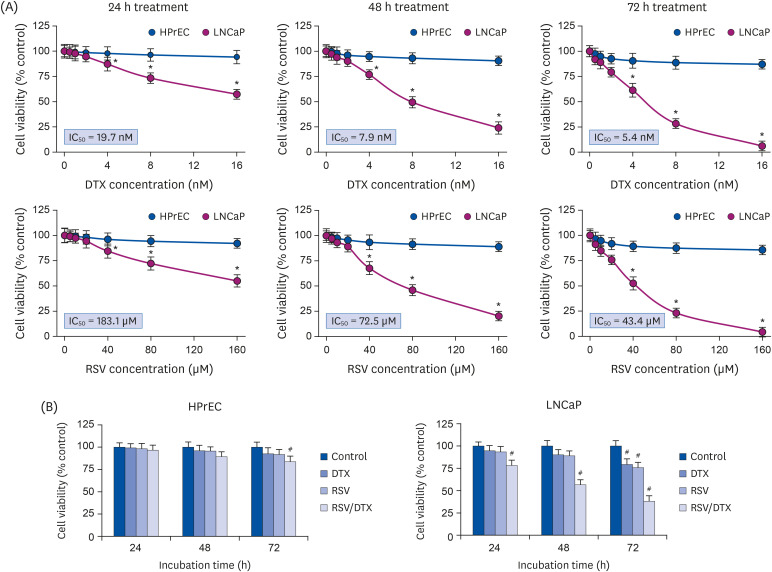 | Fig. 1Synergistic growth-inhibiting effects of RSV and DTX in LNCaP and HPrECs. (A) Cells were treated with increasing concentrations of RSV (0, 5, 10, 20, 40, 80, and 160 μM) and DTX (0, 0.5, 1, 2, 4, 8, and 16 nM) for the indicated times. (B) Cells were treated with RSV (20 μM) and DTX (2 nM) alone or in combination for 24, 48, and 72 h. The percentage of viable cells was then determined by MTT assay. Error bars represent the mean ± SD for 3 independent experiments.RSV, resveratrol; DTX, docetaxel; RSV/DTX, the combination of resveratrol and docetaxel; HPrEC, human prostate epithelial cell.
*P < 0.05 compared with respective HPrEC cells; #P < 0.05 compared with respective untreated cells.
|
Resveratrol alone or in combination with DTX induces cell cycle transition delay at G2/M phase and DNA damage response (DDR)
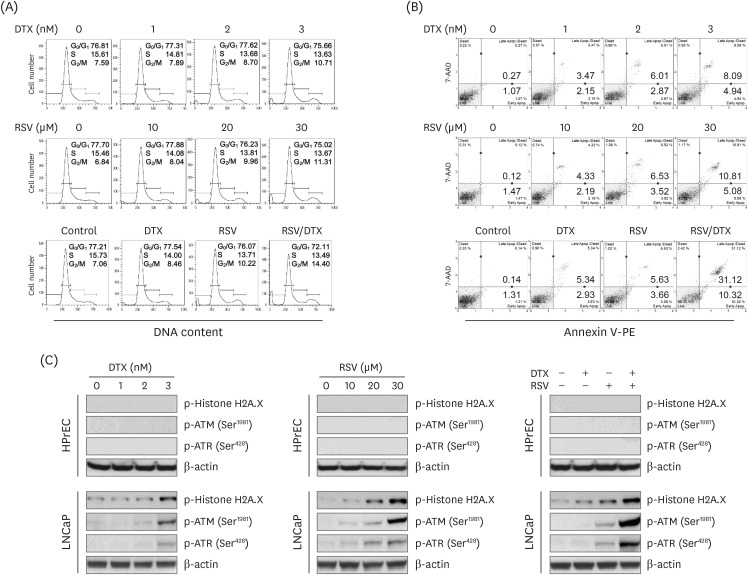 | Fig. 2Cytotoxic effects induced by RSV and DTX in LNCaP cells. Cells were treated with indicated concentrations of RSV and DTX, otherwise RSV (20 μM) and DTX (2 nM) alone or in combination for 48 h. (A) Cell cycle distribution was determined by flow cytometry following PI (20 μg/mL) staining. (B) Apoptotic cell fraction was analyzed using Annexin V-PE binding assay. (C) The levels of DNA damage response proteins were assessed by Western blotting.RSV, resveratrol; DTX, docetaxel; PI, propidium iodide; ATM, ataxia telangiectasia mutated; ATR, ataxia telangiectasia mutated and RAD3-related.
|
Synergistic cytotoxicity of resveratrol and DTX concurrently induces apoptosis and necroptosis
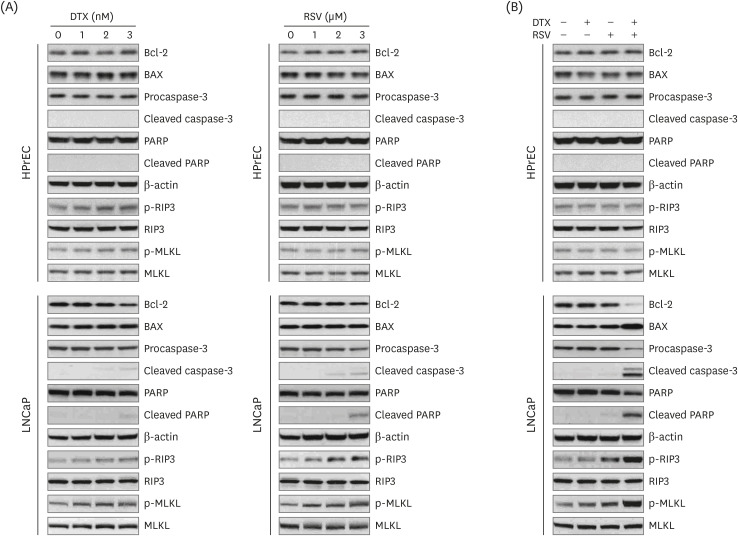 | Fig. 3Expression of apoptosis- and necroptosis-related proteins by RSV and DTX in LNCaP and HPrECs. (A) Cells were treated with increasing concentrations of RSV (0, 10, 20, and 30 μM) and DTX (0, 1, 2, and 3 nM) for 48 h. (B) Cells were treated with RSV (20 μM) and DTX (2 nM) alone or in combination for 48 h. The levels of apoptosis- and necroptosis-related proteins were assessed by Western blotting.RSV, resveratrol; DTX, docetaxel; Bcl-2, B-cell lymphoma 2; BAX, Bcl-2-associated X protein; PARP, poly ADP-ribose polymerase; RIP3, receptor-interacting protein kinase-3; MLKL, mixed lineage kinase domain-like protein; HPrEC, human prostate epithelial cell.
|
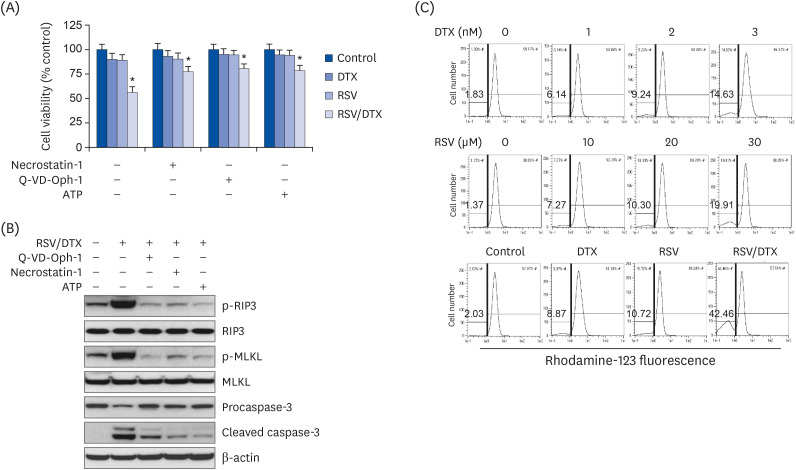 | Fig. 4Apoptosis, necroptosis, and oxidative mitochondrial dysfunction in LNCaP cell treated with RSV and DTX. Cells were pretreated with necrostatin-1 (25 μM), Q-VD-Oph-1(10 μM), and ATP (1 mM) 2 h prior to treatment with RSV (20 μM) and DTX (2 nM) in combination. (A) The percentage of viable cells was then determined by MTT assay. (B) The levels of apoptosis- and necroptosis-related proteins were assessed by Western blotting. Cells were treated with indicated concentrations of RSV and DTX, otherwise RSV (20 μM) and DTX (2 nM) alone or in combination for 48 h. (C) ΔΨm was measured after cells were stained with 30 nM rhodamine 123.RSV, resveratrol; DTX, docetaxel; RSV/DTX, the combination of resveratrol and docetaxel; RIP3, receptor-interacting protein kinase-3; MLKL, mixed lineage kinase domain-like protein; ATP, adenosine triphosphate.
*P < 0.05 vs. respective untreated cells.
|
Excessive ROS is an upstream molecule that causes synergistic cytotoxicity of resveratrol and DTX
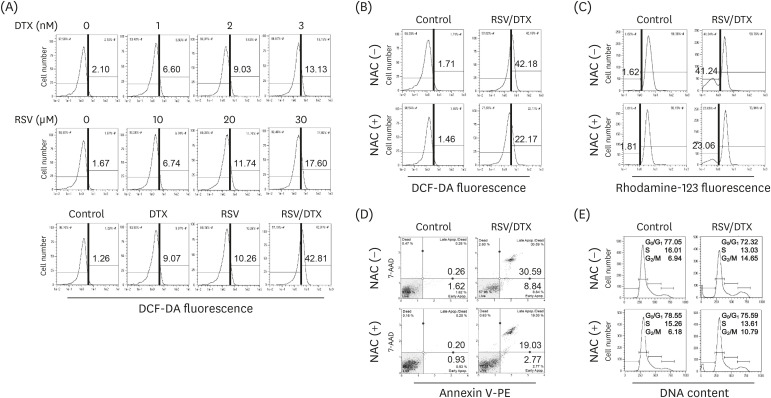 | Fig. 5Effects of ROS scavenger NAC on cytotoxicity induced by RSV and DTX in LNCaP cells. Cells were treated with indicated concentrations of RSV and DTX, otherwise RSV (20 μM) and DTX (2 nM) alone or in combination for 48 h. (A) Cellular ROS levels were measured after cells were stained with 10 μM DCF-DA. Cells were pretreated with 5 mM NAC for 2 h prior to treatment with RSV (20 μM) and DTX (2 nM) in combination. (B) Cellular ROS levels were measured by staining cells with 10 μM DCF-DA. (C) ΔΨm was measured by staining cells with 30 nM rhodamine123. (D) Apoptotic cell fraction was analyzed using Annexin V-PE binding assay. (E) Cell cycle distribution was determined by flow cytometry following PI (20 μg/mL) staining.ROS, reactive oxygen species; NAC, N-acetylcysteine; RSV, resveratrol; DTX, docetaxel; RSV/DTX, the combination of resveratrol and docetaxel; DCF-DA, 2′,7′-dichlorodihydrofluorescein diacetate.
|
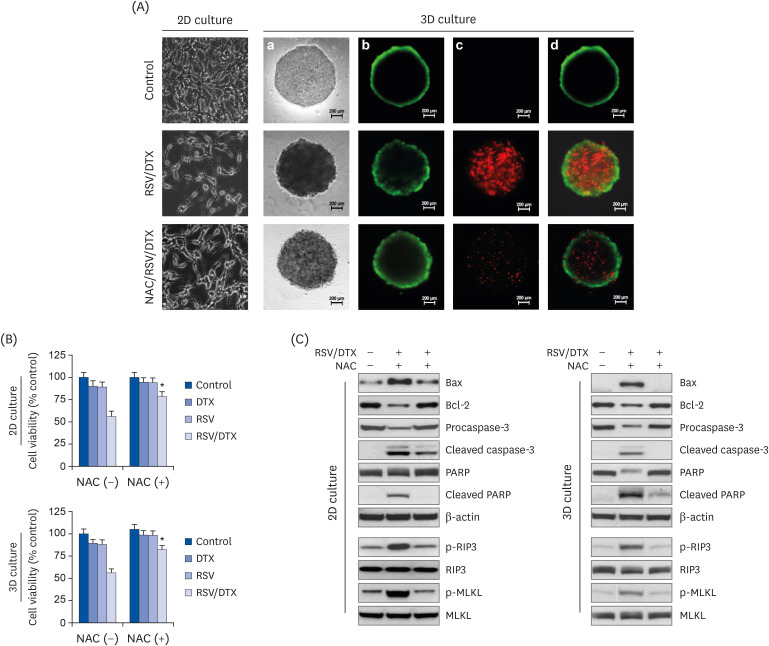 | Fig. 6Cell viability, morphology, and Western blotting analysis by RSV and DTX in 2D monolayer and 3D spheroid culture of LNCaP cells. Cells and spheroids were pretreated with or without 5 mM NAC 2 h prior to treatment with RSV (20 μM) and DTX (2 nM) in combination. (A) Vitality staining of cells (phase-contrast image of 2D monolayer cells) and spheroids [from left to right: phase-contrast image (a), fluorescent images of FDA(+) living cells in green (b), PI(+) dead cells in red (c), and merged (d)]. (B) Cells and spheroid viability were measured by MTT assay and the enhanced cell viability assay, respectively. (C) The levels of necroptosis- and apoptosis-related proteins were analyzed by Western blotting.RSV, resveratrol; 2D, 2-dimensional; 3D, 3-dimensional; NAC, N-acetylcysteine; DTX, docetaxel; RSV/DTX, the combination of resveratrol and docetaxel; Bcl-2, B-cell lymphoma 2; BAX, Bcl-2-associated X protein; PARP, poly ADP-ribose polymerase; RIP3, receptor-interacting protein kinase-3; MLKL, mixed lineage kinase domain-like protein; FDA, fluorescein diacetate; PI, propidium iodide.
*P < 0.05 vs. respective NAC-untreated cells.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download