INTRODUCTION
MATERIALS AND METHODS
Animals
Experimental protocol
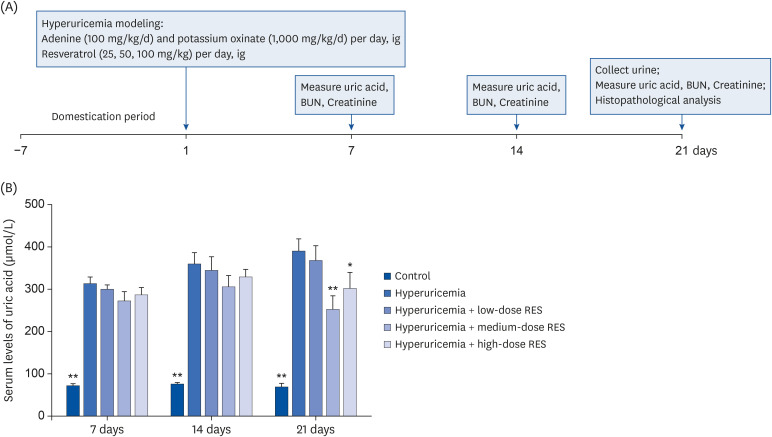 | Fig. 1The serum uric acid level in the hyperuricemia group after treatment with different dosages of resveratrol. (A) Adenine (100 mg/kg/day) and potassium oxinate (1,000 mg/kg/day) or vehicle were orally administered every morning. Low-dose (25 mg/kg/day), medium-dose (50 mg/kg/day), and high-dose (100 mg/kg/day) RES were administered every afternoon. (B) The serum levels of uric acid from the control or hyperuricemic rats treated without or with different concentrations of RES were measured. Samples were collected at 7, 14, and 21 days after treatment. Data are expressed as the mean ± SEM (n = 8).RES, resveratrol; SEM, standard error of the mean; BUN, blood urea nitrogen.
*P < 0.05, **P < 0.01 compared with the hyperuricemia group.
|
Biochemical analysis
Histopathological evaluation
Enzyme-linked immunosorbent assay (ELISA)
Statistical analysis
RESULTS
Effect of resveratrol on uric acid levels in rat serum
Effects of resveratrol on body weight and kidney indices in hyperuricemic rats
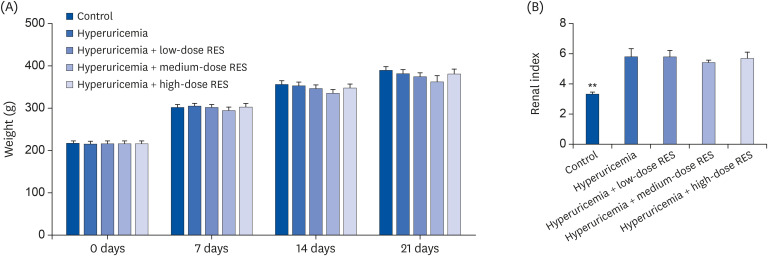 | Fig. 2The body weight and kidney indices of the hyperuricemic rats were measured after treatment with resveratrol. (A) The body weight and (B) kidney indices of the control or hyperuricemic rats treated without or with different concentrations of RES were measured at 0, 7, 14, and 21 days after treatment. Data are presented as the mean ± SEM (n = 8).RES, resveratrol; SEM, standard error of the mean.
**P < 0.01 compared with the hyperuricemia group.
|
Effects of resveratrol on the serum biochemical indicators in hyperuricemic rats
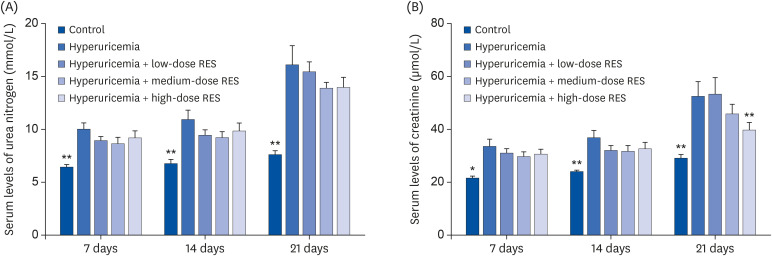 | Fig. 3The different doses of RES were administered every afternoon. (A) Serum urea nitrogen and (B) creatinine were measured to assess the renal function. Data are presented as the mean ± SEM (n = 8).RES, resveratrol; SEM, standard error of the mean.
*P < 0.05, **P < 0.01 compared with the hyperuricemia group.
|
Effects of resveratrol on urine biochemical indicators in hyperuricemic rats
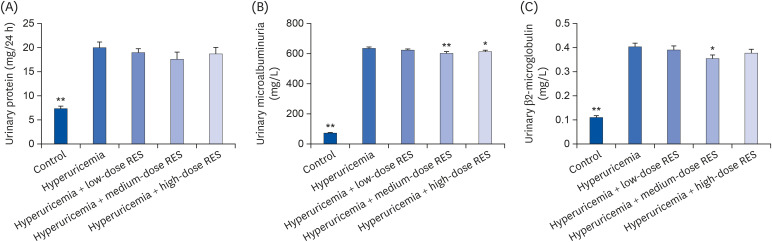 | Fig. 4The levels of (A) urine protein contents, (B) microalbumin, and (C) β2-microglobulin in urine from the control or hyperuricemic rats treated without or with different concentrations of RES were measured. Data are presented as the mean ± SEM (n = 8).RES, resveratrol; SEM, standard error of the mean.
*P < 0.05, **P < 0.01 compared with the hyperuricemia group.
|
Effects of resveratrol on morphologic changes in hyperuricemic rat kidneys
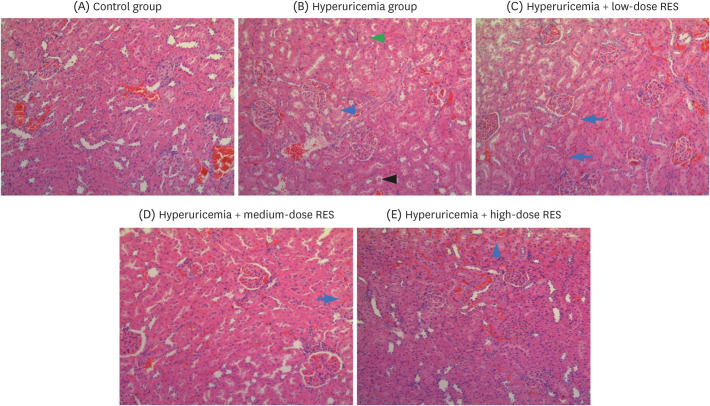 | Fig. 5RES ameliorates renal morphological changes and reduces the infiltration of inflammatory cells in the kidney. Representative photographs of H&E-stained rat renal sections at a magnification of 200×. The groups were as follows: control group, hyperuricemia group (sham), hyperuricemia with low-dose RES, hyperuricemia with medium-dose RES, and hyperuricemia with high-dose RES. The blue arrows represent eosinophilic insoluble proteins; the black arrow represents salt crystals; and the green arrow represents edema of renal tubular epithelial cells.RES, resveratrol; H&E, hematoxylin and eosin.
|
Effects of resveratrol on the inflammatory and oxidative responses
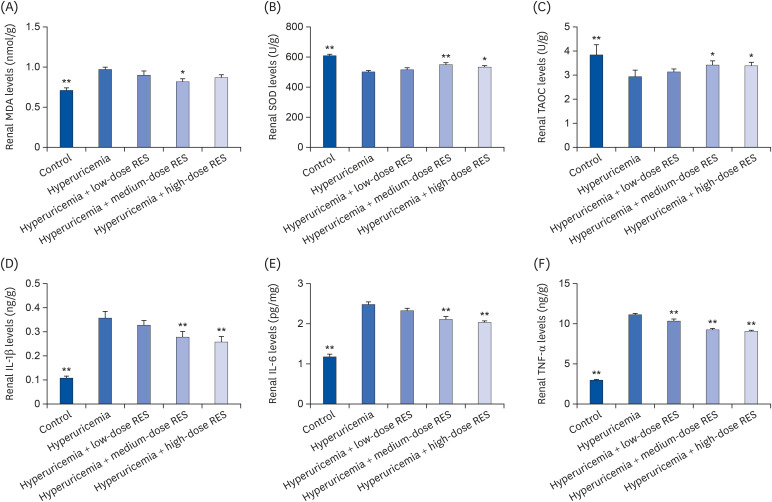 | Fig. 6RES preserves rat renal function and reduces cytokine production induced by hyperuricemia. The concentrations of (A) MDA, (B) SOD, (C) TAOC, (D) IL-1β, (E) IL-6, and (F) TNF-α in kidney tissue were measured by ELISA. The groups were as follows: control group, hyperuricemia group, hyperuricemia with low-dose RES, hyperuricemia with medium-dose RES, and hyperuricemia with high-dose RES. Data are presented as the mean ± SEM (n = 8).MDA, malondialdehyde; SOD, superoxide dismutase; TAOC, total antioxidant capacity; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; ELISA, enzyme-linked immunosorbent assay; RES, resveratrol.
*P < 0.05, **P < 0.01 compared with the hyperuricemia group.
|
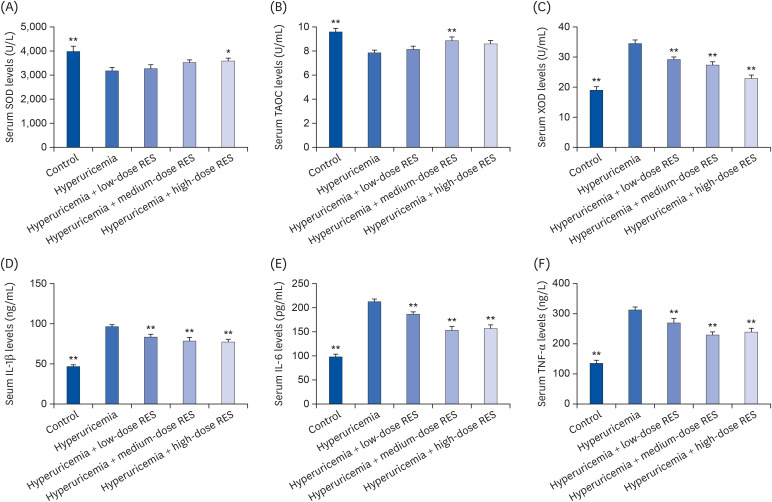 | Fig. 7The levels of (A) SOD, (B) TAOC, (C) XOD, (D) IL-1β, (E) IL-6, and (F) TNF-α in serum were measured by ELISA. The groups were as follows: control group, hyperuricemia group, hyperuricemia with low-dose RES, hyperuricemia with medium-dose RES, and hyperuricemia with high-dose RES. Data are presented as the mean ± SEM (n = 8).SOD, superoxide dismutase; TAOC, total antioxidant capacity; XOD, xanthine oxidase; IL, interleukin; TNF, tumor necrosis factor; ELISA, enzyme-linked immunosorbent assay; RES, resveratrol.
*P < 0.05, **P < 0.01 compared with the hyperuricemia group.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download