2. Chudasama YV, Gillies CL, Zaccardi F, Coles B, Davies MJ, Seidu S, et al. Impact of COVID-19 on routine care for chronic diseases: a global survey of views from healthcare professionals. Diabetes Metab Syndr. 2020; 14(5):965–967. PMID:
32604016.

3. Alene KA, Wangdi K, Clements ACA. Impact of the COVID-19 pandemic on tuberculosis control: an overview. Trop Med Infect Dis. 2020; 5(3):123.

4. McQuaid CF, McCreesh N, Read JM, Sumner T. CMMID COVID-19 Working Group, Houben RMGJ, et al. The potential impact of COVID-19-related disruption on tuberculosis burden. Eur Respir J. 2020; 56(2):2001718. PMID:
32513784.

5. Oh J, Lee JK, Schwarz D, Ratcliffe HL, Markuns JF, Hirschhorn LR. National response to COVID-19 in the Republic of Korea and lessons learned for other countries. Health Syst Reform. 2020; 6(1):e1753464. PMID:
32347772.

6. Kwak N, Hwang SS, Yim JJ. Effect of COVID-19 on tuberculosis notification, South Korea. Emerg Infect Dis. 2020; 26(10):2506–2508. PMID:
32672531.

7. Min J, Kim HW, Ko Y, Oh JY, Kang JY, Lee J, et al. Tuberculosis surveillance and monitoring under the National Public-Private Mix Tuberculosis Control Project in South Korea 2016-2017. Tuberc Respir Dis (Seoul). 2020; 83(3):218–227. PMID:
32610836.

8. Korea Centers for Disease Control & Prevention. Annual Report on the Notified Tuberculosis in Korea, 2019. Cheongju, Korea: Korea Centers for Disease Control & Prevention;2020.
9. Kang HY, Yoo H, Park W, Go U, Jeong E, Jung KS, et al. Tuberculosis Notification Completeness and Timeliness in the Republic of Korea During 2012-2014. Osong Public Health Res Perspect. 2016; 7(5):320–326. PMID:
27812491.

10. World Health Organization. Definitions and Reporting Framework for Tuberculosis – 2013 Revision. Geneva, Switzerland: World Health Organization;2013.
11. Korea Centers for Disease Control & Prevention. Korean Guidelines for Tuberculosis. 4th ed. Cheongju, Korea: Joint Committee for the Revision of Korean Guidelines for Tuberculosis and Korea Centers for Disease Control & Prevention;2020.
12. Kim M, Park K, Kim Y, Kim Y, Yeom H, Hwang I, et al. Weekly report on the COVID-19 situation in the Republic of Korea (as of July 4, 2020). Public Health Wkly Rep. 2020; 13(28):2032–2046.
14. Magassouba AS, Diallo BD, Camara LM, Sow K, Camara S, Bah B, et al. Impact of the Ebola virus disease outbreak (2014–2016) on tuberculosis surveillance activities by Guinea's National Tuberculosis Control Program: a time series analysis. BMC Public Health. 2020; 20(1):1200. PMID:
32753044.

15. Desta KT, Kessely DB, Daboi JG. Evaluation of the performance of the National Tuberculosis Program of Liberia during the 2014–2015 Ebola outbreak. BMC Public Health. 2019; 19(1):1221. PMID:
31484578.

16. Parpia AS, Ndeffo-Mbah ML, Wenzel NS, Galvani AP. Effects of response to 2014–2015 Ebola outbreak on deaths from Malaria, HIV/AIDS, and tuberculosis, West Africa. Emerg Infect Dis. 2016; 22(3):433–441. PMID:
26886846.

17. Visca D, Tiberi S, Pontali E, Spanevello A, Migliori GB. Tuberculosis in the time of COVID-19: quality of life and digital innovation. Eur Respir J. 2020; 56(2):2001998. PMID:
32513783.

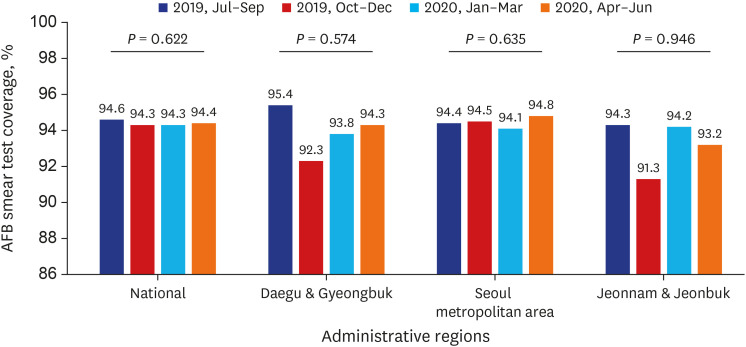
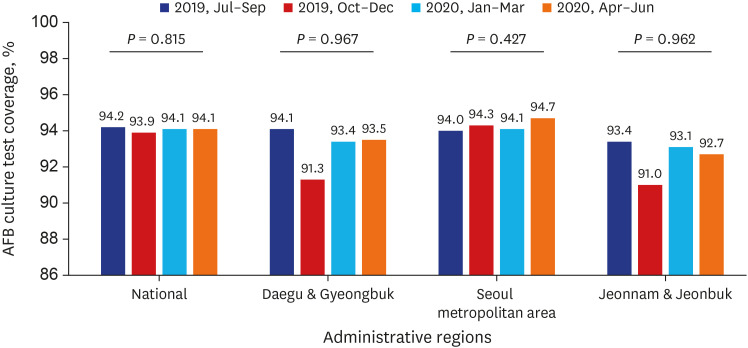
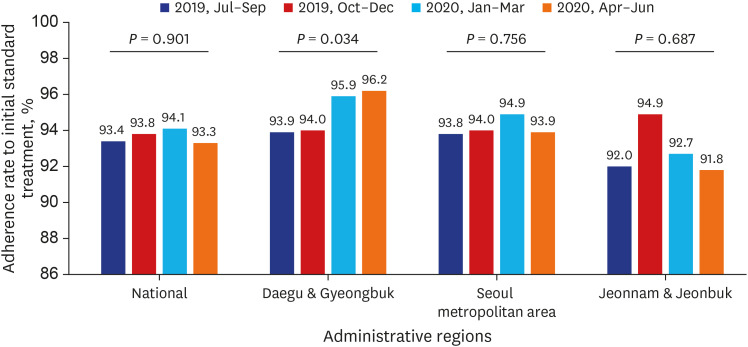
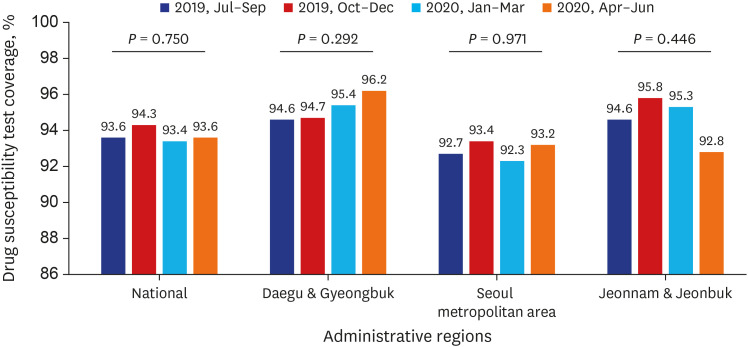
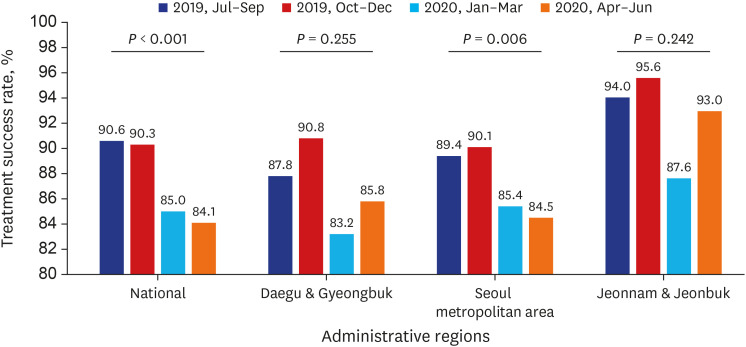
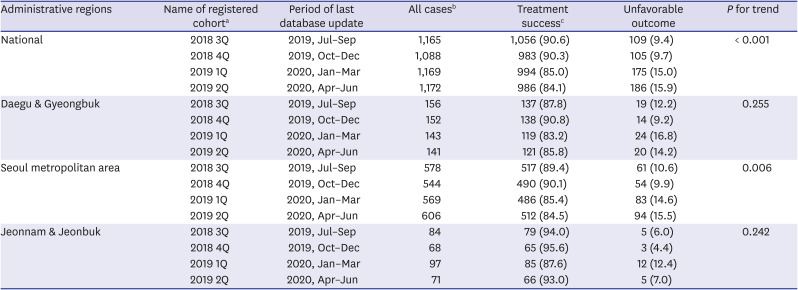
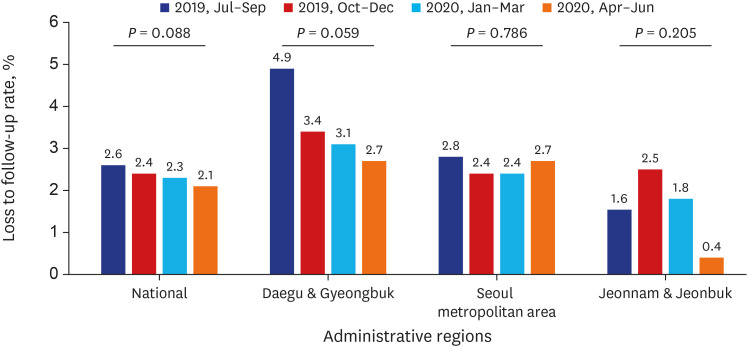
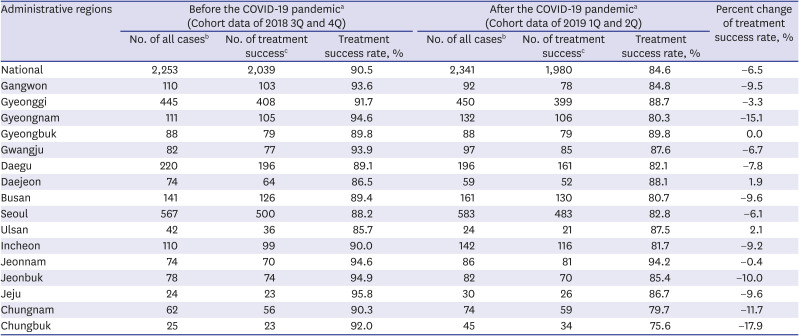




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download