1. Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006; 20:633–649. PMID:
16997150.

2. Jung KW, Won YJ, Kong HJ, Lee ES. Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat. 2018; 50:303–316. PMID:
29566481.

3. Akagi T, Shiraishi N, Kitano S. Lymph node metastasis of gastric cancer. Cancers (Basel). 2011; 3:2141–2159. PMID:
24212800.

4. Werner M, Becker KF, Keller G, Höfler H. Gastric adenocarcinoma: pathomorphology and molecular pathology. J Cancer Res Clin Oncol. 2001; 127:207–216. PMID:
11315254.

5. Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003; 3:453–458. PMID:
12778135.

6. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011; 331:1559–1564. PMID:
21436443.

7. Jang IS, Park JW, Jo EB, Cho CK, Lee YW, Yoo HS, Park J, Kim J, Jang BC, Choi JS. Growth inhibitory and apoptosis-inducing effects of allergen-free
Rhus verniciflua Stokes extract on A549 human lung cancer cells. Oncol Rep. 2016; 36:3037–3043. PMID:
27667098.
8. Lee KW, Um ES, Jung BB, Choi ES, Kim EY, Lee S, Jang E, Lee JH, Kim Y.
Rhus verniciflua Stokes extract induces inhibition of cell growth and apoptosis in human chronic myelogenous leukemia K562 cells. Oncol Rep. 2018; 39:1141–1147. PMID:
29328387.
9. Jang H, Lee JW, Lee C, Jin Q, Lee MK, Lee CK, Lee MK, Hwang BY. Flavonol glycosides from the aerial parts of
Gynostemma pentaphyllum and their antioxidant activity. Arch Pharm Res. 2016; 39:1232–1236. PMID:
27384065.
10. Jang HS, Kook SH, Son YO, Kim JG, Jeon YM, Jang YS, Choi KC, Kim J, Han SK, Lee KY, Park BK, Cho NP, Lee JC. Flavonoids purified from
Rhus verniciflua Stokes actively inhibit cell growth and induce apoptosis in human osteosarcoma cells. Biochim Biophys Acta. 2005; 1726:309–316. PMID:
16213662.
11. Jung CH, Jun CY, Lee S, Park CH, Cho K, Ko SG.
Rhus verniciflua Stokes extract: radical scavenging activities and protective effects on H2O2-induced cytotoxicity in macrophage RAW 264.7 cell lines. Biol Pharm Bull. 2006; 29:1603–1607. PMID:
16880612.
12. Jung CH, Kim JH, Hong MH, Seog HM, Oh SH, Lee PJ, Kim GJ, Kim HM, Um JY, Ko SG. Phenolic-rich fraction from
Rhus verniciflua Stokes (RVS) suppress inflammatory response via NF-κB and JNK pathway in lipopolysaccharide-induced RAW 264.7 macrophages. J Ethnopharmacol. 2007; 110:490–497. PMID:
17112694.
13. Kitts DD, Lim KT. Antitumorigenic and cytotoxic properties of an ethanol extract derived from
Rhus verniciflua Stokes (RVS). J Toxicol Environ Health A. 2001; 64:357–371. PMID:
11693493.
14. Lee JC, Lim KT, Jang YS. Identification of
Rhus verniciflua Stokes compounds that exhibit free radical scavenging and anti-apoptotic properties. Biochim Biophys Acta. 2002; 1570:181–191. PMID:
12020808.
15. Lee JC, Lee KY, Kim J, Na CS, Jung NC, Chung GH, Jang YS. Extract from
Rhus verniciflua Stokes is capable of inhibiting the growth of human lymphoma cells. Food Chem Toxicol. 2004; 42:1383–1388. PMID:
15234068.
16. Lee SH, Choi WC, Kim KS, Park JW, Lee SH, Yoon SW. Shrinkage of gastric cancer in an elderly patient who received
Rhus verniciflua Stokes extract. J Altern Complement Med. 2010; 16:497–500. PMID:
20423218.
17. Lee SO, Kim SJ, Kim JS, Ji H, Lee EO, Lee HJ. Comparison of the main components and bioactivity of
Rhus verniciflua Stokes extracts by different detoxification processing methods. BMC Complement Altern Med. 2018; 18:242. PMID:
30165848.

18. Lim KT, Hu C, Kitts DD. Antioxidant activity of a
Rhus verniciflua Stokes ethanol extract. Food Chem Toxicol. 2001; 39:229–237. PMID:
11278054.
19. Choi W, Jung H, Kim K, Lee S, Yoon S, Park J, Kim S, Cheon S, Eo W, Lee S.
Rhus verniciflua Stokes against advanced cancer: a perspective from the Korean Integrative Cancer Center. J Biomed Biotechnol. 2012; 2012:874276. PMID:
22174564.
20. Kim JH, Go HY, Jin DH, Kim HP, Hong MH, Chung WY, Park JH, Jang JB, Jung H, Shin YC, Kim SH, Ko SG. Inhibition of the PI3K-Akt/PKB survival pathway enhanced an ethanol extract of
Rhus verniciflua Stokes-induced apoptosis via a mitochondrial pathway in AGS gastric cancer cell lines. Cancer Lett. 2008; 265:197–205. PMID:
18378393.
21. Lee JO, Moon JW, Lee SK, Kim SM, Kim N, Ko SG, Kim HS, Park SH.
Rhus verniciflua extract modulates survival of MCF-7 breast cancer cells through the modulation of AMPK-pathway. Biol Pharm Bull. 2014; 37:794–801. PMID:
24553147.
22. Kim JH, Kim HP, Jung CH, Hong MH, Hong MC, Bae HS, Lee SD, Park SY, Park JH, Ko SG. Inhibition of cell cycle progression via p27Kip1 upregulation and apoptosis induction by an ethanol extract of
Rhus verniciflua Stokes in AGS gastric cancer cells. Int J Mol Med. 2006; 18:201–208. PMID:
16786174.
23. Son YO, Lee KY, Lee JC, Jang HS, Kim JG, Jeon YM, Jang YS. Selective antiproliferative and apoptotic effects of flavonoids purified from
Rhus verniciflua Stokes on normal versus transformed hepatic cell lines. Toxicol Lett. 2005; 155:115–125. PMID:
15585366.
24. Kim EJ, Holthuizen PE, Park HS, Ha YL, Jung KC, Park JH. Trans-10,cis-12-conjugated linoleic acid inhibits Caco-2 colon cancer cell growth. Am J Physiol Gastrointest Liver Physiol. 2002; 283:G357–67. PMID:
12121883.

25. Cho HJ, Kim WK, Kim EJ, Jung KC, Park S, Lee HS, Tyner AL, Park JH. Conjugated linoleic acid inhibits cell proliferation and ErbB3 signaling in HT-29 human colon cell line. Am J Physiol Gastrointest Liver Physiol. 2003; 284:G996–1005. PMID:
12571082.
26. Kang SH, Hwang IH, Son E, Cho CK, Choi JS, Park SJ, Jang BC, Lee KB, Lee ZW, Lee JH, Yoo HS, Jang IS. Allergen-removed
Rhus verniciflua extract induces ovarian cancer cell death via JNK activation. Am J Chin Med. 2016; 44:1719–1735. PMID:
27848251.
27. Jung MH, Lee SH, Ahn EM, Lee YM. Decursin and decursinol angelate inhibit VEGF-induced angiogenesis via suppression of the VEGFR-2-signaling pathway. Carcinogenesis. 2009; 30:655–661. PMID:
19228635.

28. Kang KA, Piao MJ, Madduma Hewage SR, Ryu YS, Oh MC, Kwon TK, Chae S, Hyun JW. Fisetin induces apoptosis and endoplasmic reticulum stress in human non-small cell lung cancer through inhibition of the MAPK signaling pathway. Tumour Biol. 2016; 37:9615–9624. PMID:
26797785.

29. Kook SH, Son YO, Chung SW, Lee SA, Kim JG, Jeon YM, Lee JC. Caspase-independent death of human osteosarcoma cells by flavonoids is driven by p53-mediated mitochondrial stress and nuclear translocation of AIF and endonuclease G. Apoptosis. 2007; 12:1289–1298. PMID:
17356895.

30. Lee JC, Kim J, Jang YS. Ethanol-eluted extract of
Rhus verniciflua Stokes inhibits cell growth and induces apoptosis in human lymphoma cells. J Biochem Mol Biol. 2003; 36:337–343. PMID:
12895289.
31. Chae J, Lee S, Lee S. Potential Efficacy of Allergen Removed Rhus Verniciflua Stokes Extract to Maintain Progression-Free Survival of Patients With Advanced Hepatobiliary Cancer. Explore (NY). 2018; 14:300–304. PMID:
29803518.
32. Kim KS, Jung HS, Choi WC, Eo WK, Cheon SH, Cheon SH. A case of recurred hepatocellular carcinoma refractory to doxorubicin after liver transplantation showing response to herbal medicine product,
Rhus verniciflua Stokes extract. Integr Cancer Ther. 2010; 9:100–104. PMID:
20308087.
33. Lee SH, Choi WC, Yoon SW. Impact of standardized
Rhus verniciflua Stokes extract as complementary therapy on metastatic colorectal cancer: a Korean single-center experience. Integr Cancer Ther. 2009; 8:148–152. PMID:
19679623.
34. Lee SH, Kim KS, Choi WC, Yoon SW. Successful outcome of advanced pulmonary adenocarcinoma with malignant pleural effusion by the standardized
Rhus verniciflua Stokes extract: a case study. Explore (NY). 2009; 5:242–244. PMID:
19608113.
35. Lee SK, Jung HS, Eo WK, Lee SY, Kim SH, Shim BS.
Rhus verniciflua Stokes extract as a potential option for treatment of metastatic renal cell carcinoma: report of two cases. Ann Oncol. 2010; 21:1383–1385. PMID:
20363807.
36. Choi HS, Yeo SH, Jeong ST, Choi JH, Park HS, Kim MK. Preparation and characterization of urushiol free fermented Rhus verniciflua stem bark (FRVSB) extracts. Korean J Food Sci Technol. 2012; 44:173–178.
37. Kobayashi S, Ikeda R, Oyabu H, Tanaka H, Uyama H. Artificial urushi: design, synthesis, and enzymatic curing of new urushiol analogues. Chem Lett. 2000; 29:1214–1215.

38. Choi HS, Kim MK, Park HS, Yun SE, Mun SP, Kim JS, Sapkota K, Kim S, Kim TY, Kim SJ. Biological detoxification of lacquer tree (Rhus verniciflua Stokes) stem bark by mushroom species. Food Sci Biotechnol. 2007; 16:935–942.
39. Kim JH, Jung CH, Jang BH, Go HY, Park JH, Choi YK, Hong SI, Shin YC, Ko SG. Selective cytotoxic effects on human cancer cell lines of phenolic-rich ethyl-acetate fraction from
Rhus verniciflua Stokes. Am J Chin Med. 2009; 37:609–620. PMID:
19606519.
40. Kim JH, Shin YC, Ko SG. Integrating traditional medicine into modern inflammatory diseases care: multitargeting by
Rhus verniciflua Stokes. Mediators Inflamm. 2014; 2014:154561. PMID:
25024508.
41. Jang JY, Shin H, Lim JW, Ahn JH, Jo YH, Lee KY, Hwang BY, Jung SJ, Kang SY, Lee MK. Comparison of antibacterial activity and phenolic constituents of bark, lignum, leaves and fruit of
Rhus verniciflua
. PLoS One. 2018; 13:e0200257. PMID:
30044823.
42. Khan N, Adhami VM, Afaq F, Mukhtar H. Butein induces apoptosis and inhibits prostate tumor growth
in vitro and
in vivo
. Antioxid Redox Signal. 2012; 16:1195–1204. PMID:
22114764.
43. Samoszuk M, Tan J, Chorn G. The chalcone butein from
Rhus verniciflua Stokes inhibits clonogenic growth of human breast cancer cells co-cultured with fibroblasts. BMC Complement Altern Med. 2005; 5:5. PMID:
15757513.

44. Yit CC, Das NP. Cytotoxic effect of butein on human colon adenocarcinoma cell proliferation. Cancer Lett. 1994; 82:65–72. PMID:
8033070.

45. Park BC, Lee YS, Park HJ, Kwak MK, Yoo BK, Kim JY, Kim JA. Protective effects of fustin, a flavonoid from
Rhus verniciflua Stokes, on 6-hydroxydopamine-induced neuronal cell death. Exp Mol Med. 2007; 39:316–326. PMID:
17603285.
46. Liu T, Li Z, Li R, Cui Y, Zhao Y, Yu Z. Composition analysis and antioxidant activities of the
Rhus typhina L. stem. J Pharm Anal. 2019; 9:332–338. PMID:
31929942.
47. Grynkiewicz G, Demchuk OM. New perspectives for fisetin. Front Chem. 2019; 7:697. PMID:
31750288.

48. Lee JD, Huh JE, Jeon G, Yang HR, Woo HS, Choi DY, Park DS. Flavonol-rich RVHxR from
Rhus verniciflua Stokes and its major compound fisetin inhibits inflammation-related cytokines and angiogenic factor in rheumatoid arthritic fibroblast-like synovial cells and
in vivo models. Int Immunopharmacol. 2009; 9:268–276. PMID:
19111632.
49. Lall RK, Adhami VM, Mukhtar H. Dietary flavonoid fisetin for cancer prevention and treatment. Mol Nutr Food Res. 2016; 60:1396–1405. PMID:
27059089.

50. Hostetler GL, Ralston RA, Schwartz SJ. Flavones: food sources, bioavailability, metabolism, and bioactivity. Adv Nutr. 2017; 8:423–435. PMID:
28507008.

51. Kashyap D, Sharma A, Sak K, Tuli HS, Buttar HS, Bishayee A. Fisetin: a bioactive phytochemical with potential for cancer prevention and pharmacotherapy. Life Sci. 2018; 194:75–87. PMID:
29225112.

52. Wang TY, Li Q, Bi KS. Bioactive flavonoids in medicinal plants: structure, activity and biological fate. Asian J Pharm Sci. 2018; 13:12–23. PMID:
32104374.

53. Syed DN, Adhami VM, Khan MI, Mukhtar H. Inhibition of Akt/mTOR signaling by the dietary flavonoid fisetin. Anticancer Agents Med Chem. 2013; 13:995–1001. PMID:
23293889.

54. Gupta SC, Tyagi AK, Deshmukh-Taskar P, Hinojosa M, Prasad S, Aggarwal BB. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch Biochem Biophys. 2014; 559:91–99. PMID:
24946050.

55. Salerno S, Da Settimo F, Taliani S, Simorini F, La Motta C, Fornaciari G, Marini AM. Recent advances in the development of dual topoisomerase I and II inhibitors as anticancer drugs. Curr Med Chem. 2010; 17:4270–4290. PMID:
20939813.

56. Freije JM, Balbín M, Pendás AM, Sánchez LM, Puente XS, López-Otín C. Matrix metalloproteinases and tumor progression. Adv Exp Med Biol. 2003; 532:91–107. PMID:
12908552.

57. Tang L, Han X. The urokinase plasminogen activator system in breast cancer invasion and metastasis. Biomed Pharmacother. 2013; 67:179–182. PMID:
23201006.

58. Andreasen PA, Kjøller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer. 1997; 72:1–22. PMID:
9212216.

59. Yuan ZL, Guan YJ, Wang L, Wei W, Kane AB, Chin YE. Central role of the threonine residue within the p+1 loop of receptor tyrosine kinase in STAT3 constitutive phosphorylation in metastatic cancer cells. Mol Cell Biol. 2004; 24:9390–9400. PMID:
15485908.

60. Akira S, Nishio Y, Inoue M, Wang XJ, Wei S, Matsusaka T, Yoshida K, Sudo T, Naruto M, Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994; 77:63–71. PMID:
7512451.

61. Lim CP, Cao X. Structure, function, and regulation of STAT proteins. Mol Biosyst. 2006; 2:536–550. PMID:
17216035.

62. Silva CM. Role of STATs as downstream signal transducers in Src family kinase-mediated tumorigenesis. Oncogene. 2004; 23:8017–8023. PMID:
15489919.

63. Tkach M, Rosemblit C, Rivas MA, Proietti CJ, Díaz Flaqué MC, Mercogliano MF, Beguelin W, Maronna E, Guzmán P, Gercovich FG, Deza EG, Elizalde PV, Schillaci R. p42/p44 MAPK-mediated Stat3Ser727 phosphorylation is required for progestin-induced full activation of Stat3 and breast cancer growth. Endocr Relat Cancer. 2013; 20:197–212. PMID:
23329648.

64. Alvarez JV, Greulich H, Sellers WR, Meyerson M, Frank DA. Signal transducer and activator of transcription 3 is required for the oncogenic effects of non-small-cell lung cancer-associated mutations of the epidermal growth factor receptor. Cancer Res. 2006; 66:3162–3168. PMID:
16540667.

65. Klampfer L. Signal transducers and activators of transcription (STATs): Novel targets of chemopreventive and chemotherapeutic drugs. Curr Cancer Drug Targets. 2006; 6:107–121. PMID:
16529541.

66. Kusaba T, Nakayama T, Yamazumi K, Yakata Y, Yoshizaki A, Inoue K, Nagayasu T, Sekine I. Activation of STAT3 is a marker of poor prognosis in human colorectal cancer. Oncol Rep. 2006; 15:1445–1451. PMID:
16685378.

67. Yin W, Cheepala S, Roberts JN, Syson-Chan K, DiGiovanni J, Clifford JL. Active Stat3 is required for survival of human squamous cell carcinoma cells in serum-free conditions. Mol Cancer. 2006; 5:15. PMID:
16603078.
68. Vlahopoulos SA. Aberrant control of NF-κB in cancer permits transcriptional and phenotypic plasticity, to curtail dependence on host tissue: molecular mode. Cancer Biol Med. 2017; 14:254–270. PMID:
28884042.
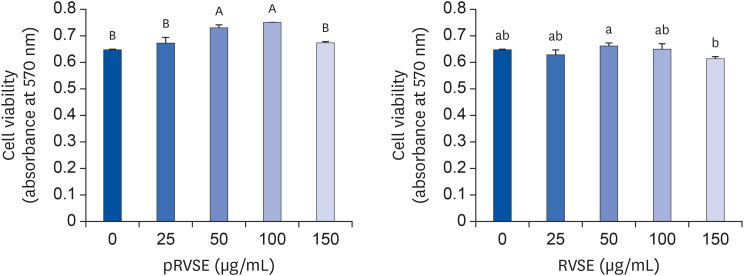
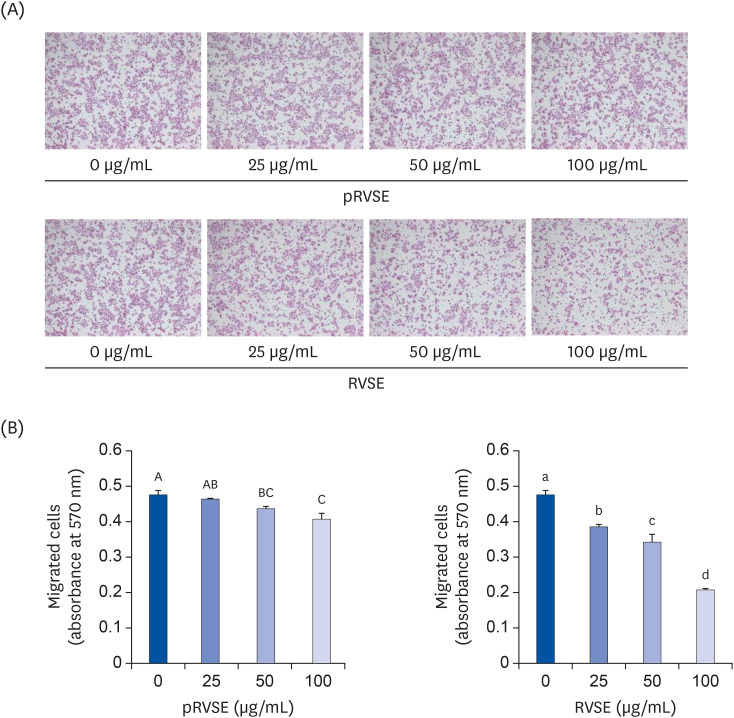
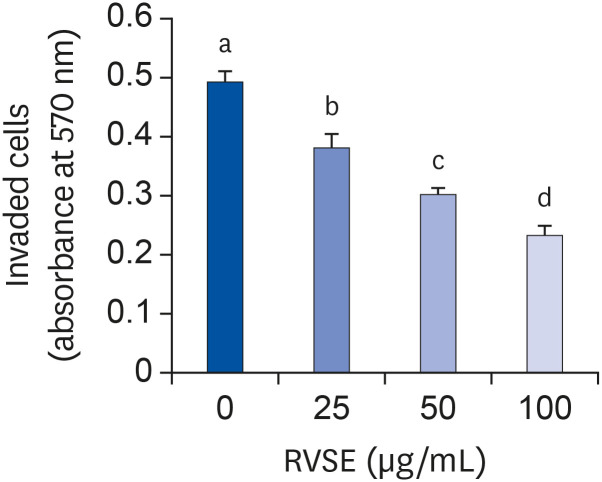
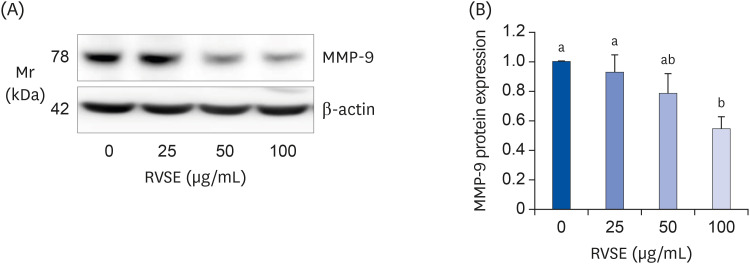




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download