1. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015; 62(1 Suppl):S47–S64. PMID:
25920090.

2. Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem. 2009; 42:1331–1346. PMID:
19501581.

3. Komiya H, Mori Y, Yokose T, Kurokawa N, Horie N, Tajima N. Effect of intramuscular fat difference on glucose and insulin reaction in oral glucose tolerance test. J Atheroscler Thromb. 2006; 13:136–142. PMID:
16835468.

4. Therkelsen KE, Pedley A, Speliotes EK, Massaro JM, Murabito J, Hoffmann U, et al. Intramuscular fat and associations with metabolic risk factors in the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2013; 33:863–870. PMID:
23349188.

5. Krssak M, Roden M. The role of lipid accumulation in liver and muscle for insulin resistance and type 2 diabetes mellitus in humans. Rev Endocr Metab Disord. 2004; 5:127–134. PMID:
15041788.

6. Tilg H, Hotamisligil GS. Nonalcoholic fatty liver disease: cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology. 2006; 131:934–945. PMID:
16952562.

7. Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008; 103:1372–1379. PMID:
18510618.

8. Lim S, Meigs JB. Links between ectopic fat and vascular disease in humans. Arterioscler Thromb Vasc Biol. 2014; 34:1820–1826. PMID:
25035342.

9. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010; 363:1341–1350. PMID:
20879883.

10. Koo BK, Allison MA, Criqui MH, Denenberg JO, Wright CM. The association between liver fat and systemic calcified atherosclerosis. J Vasc Surg. 2020; 71:204–211. PMID:
31153702.

11. Chau YY, Bandiera R, Serrels A, Martinez-Estrada OM, Qing W, Lee M, et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014; 16:367–375. PMID:
24609269.

12. Roever L, Resende ES, Veloso FC, Diniz AL, Penha-Silva N, Casella-Filho A, et al. Perirenal fat and association with metabolic risk factors: the Uberlandia Heart Study. Medicine (Baltimore). 2015; 94:e1105.
13. De Pergola G, Campobasso N, Nardecchia A, Triggiani V, Caccavo D, Gesualdo L, et al. Para- and perirenal ultrasonographic fat thickness is associated with 24-hours mean diastolic blood pressure levels in overweight and obese subjects. BMC Cardiovasc Disord. 2015; 15:108. PMID:
26419359.

14. Lamacchia O, Nicastro V, Camarchio D, Valente U, Grisorio R, Gesualdo L, et al. Para- and perirenal fat thickness is an independent predictor of chronic kidney disease, increased renal resistance index and hyperuricaemia in type-2 diabetic patients. Nephrol Dial Transplant. 2011; 26:892–898. PMID:
20798120.

15. Dwyer TM, Mizelle HL, Cockrell K, Buhner P. Renal sinus lipomatosis and body composition in hypertensive, obese rabbits. Int J Obes Relat Metab Disord. 1995; 19:869–874. PMID:
8963354.
16. Spit KA, Muskiet MH, Tonneijck L, Smits MM, Kramer MH, Joles JA, et al. Renal sinus fat and renal hemodynamics: a cross-sectional analysis. MAGMA. 2020; 33:73–80. PMID:
31471702.

17. Favre G, Grangeon-Chapon C, Raffaelli C, Francois-Chalmin F, Iannelli A, Esnault V. Perirenal fat thickness measured with computed tomography is a reliable estimate of perirenal fat mass. PLoS One. 2017; 12:e0175561. PMID:
28423008.

18. Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014; 371:1131–1141. PMID:
25229917.

19. Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1998; 158:1855–1867. PMID:
9759681.
20. Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004; 24:331–336. PMID:
14656730.

21. Hughes-Austin JM, Dominguez A 3rd, Allison MA, Wassel CL, Rifkin DE, Morgan CG, et al. Relationship of coronary calcium on standard chest CT scans with mortality. JACC Cardiovasc Imaging. 2016; 9:152–159. PMID:
26777213.

22. Lin TC, Wright CM, Criqui MH, Allison MA. Superior mesenteric artery calcification is associated with cardiovascular risk factors, systemic calcified atherosclerosis, and increased mortality. J Vasc Surg. 2018; 67:1484–1490. PMID:
29103930.

23. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990; 15:827–832. PMID:
2407762.

24. Eisner BH, Zargooshi J, Berger AD, Cooperberg MR, Doyle SM, Sheth S, et al. Gender differences in subcutaneous and perirenal fat distribution. Surg Radiol Anat. 2010; 32:879–882. PMID:
20607260.

25. Hervochon R, Bobbio A, Guinet C, Mansuet-Lupo A, Rabbat A, Regnard JF, et al. Body mass index and total psoas area affect outcomes in patients undergoing pneumonectomy for cancer. Ann Thorac Surg. 2017; 103:287–295. PMID:
27659598.

26. Portney LG, Watkins MP. Foundations of clinical research: applications to practice. 2nd ed. Upper Saddle River: Prentice Hall Health;2000.
27. Rifkin DE, Ix JH, Wassel CL, Criqui MH, Allison MA. Renal artery calcification and mortality among clinically asymptomatic adults. J Am Coll Cardiol. 2012; 60:1079–1085. PMID:
22939556.

28. Wu H, Cheng XW, Hao C, Zhang Z, Yao H, Murohara T, et al. Regulation of apelin and its receptor expression in adipose tissues of obesity rats with hypertension and cultured 3T3-L1 adipocytes. Exp Anim. 2014; 63:257–267. PMID:
24770651.
29. Kwon EY, Shin SK, Cho YY, Jung UJ, Kim E, Park T, et al. Time-course microarrays reveal early activation of the immune transcriptome and adipokine dysregulation leads to fibrosis in visceral adipose depots during diet-induced obesity. BMC Genomics. 2012; 13:450. PMID:
22947075.

30. Hoogduijn MJ, Crop MJ, Peeters AM, van Osch GJ, Balk AH, Ijzermans JN, et al. Human heart, spleen, and perirenal fat-derived mesenchymal stem cells have immunomodulatory capacities. Stem Cells Dev. 2007; 16:597–604. PMID:
17784833.

31. Niijima A. Reflex effects from leptin sensors in the white adipose tissue of the epididymis to the efferent activity of the sympathetic and vagus nerve in the rat. Neurosci Lett. 1999; 262:125–128. PMID:
10203247.

32. Liu BX, Sun W, Kong XQ. Perirenal fat: a unique fat pad and potential target for cardiovascular disease. Angiology. 2019; 70:584–593. PMID:
30301366.

33. Chughtai HL, Morgan TM, Rocco M, Stacey B, Brinkley TE, Ding J, et al. Renal sinus fat and poor blood pressure control in middle-aged and elderly individuals at risk for cardiovascular events. Hypertension. 2010; 56:901–906. PMID:
20837881.

34. Foster MC, Hwang SJ, Porter SA, Massaro JM, Hoffmann U, Fox CS. Fatty kidney, hypertension, and chronic kidney disease: the Framingham Heart Study. Hypertension. 2011; 58:784–790. PMID:
21931075.
35. Lemieux I, Lamarche B, Couillard C, Pascot A, Cantin B, Bergeron J, et al. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec Cardiovascular Study. Arch Intern Med. 2001; 161:2685–2692. PMID:
11732933.
36. Lamarche B, Moorjani S, Lupien PJ, Cantin B, Bernard PM, Dagenais GR, et al. Apolipoprotein A-I and B levels and the risk of ischemic heart disease during a five-year follow-up of men in the Quebec cardiovascular study. Circulation. 1996; 94:273–278. PMID:
8759066.

37. Cui Y, Blumenthal RS, Flaws JA, Whiteman MK, Langenberg P, Bachorik PS, et al. Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch Intern Med. 2001; 161:1413–1419. PMID:
11386890.

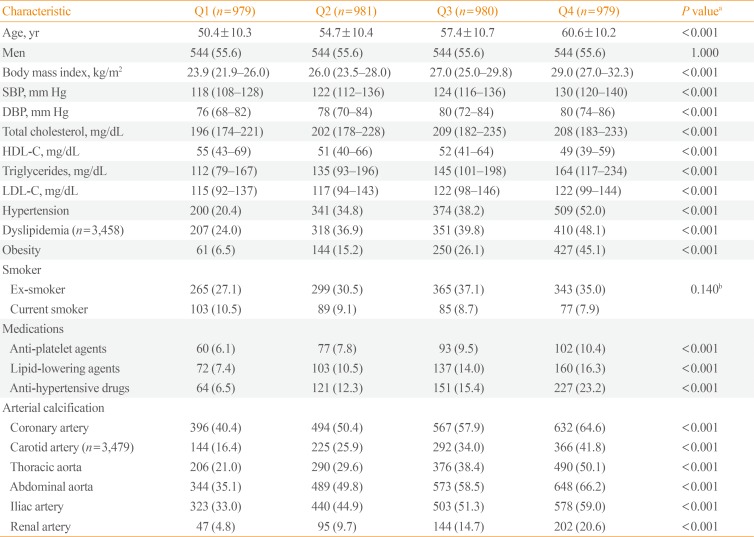
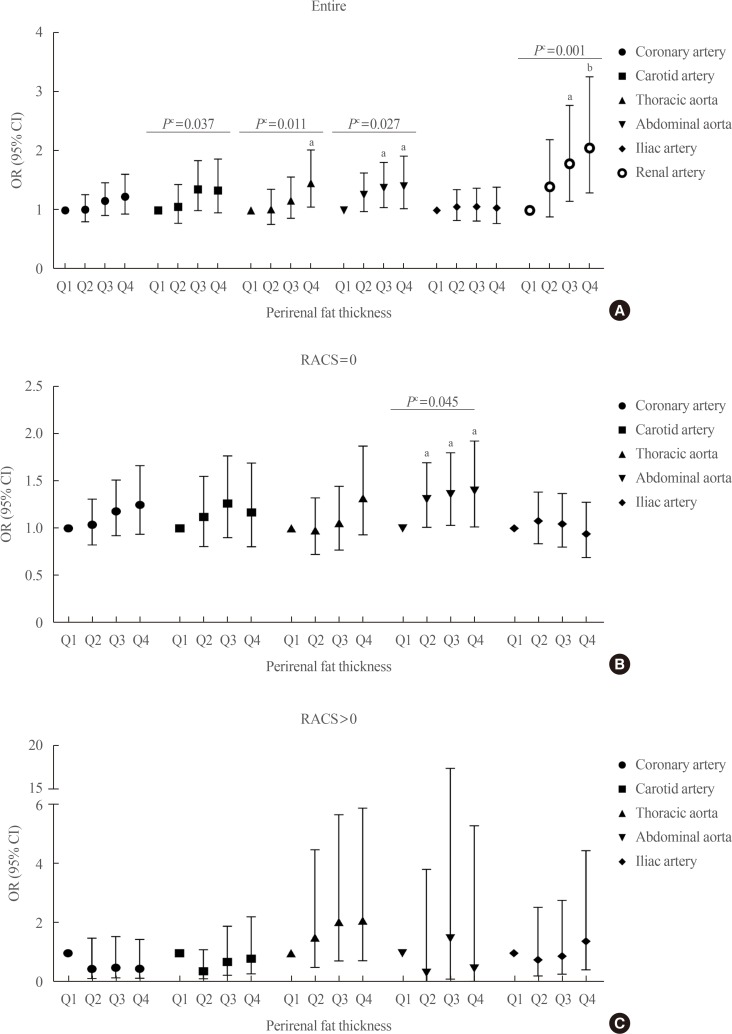
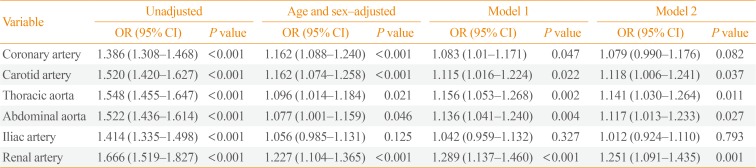
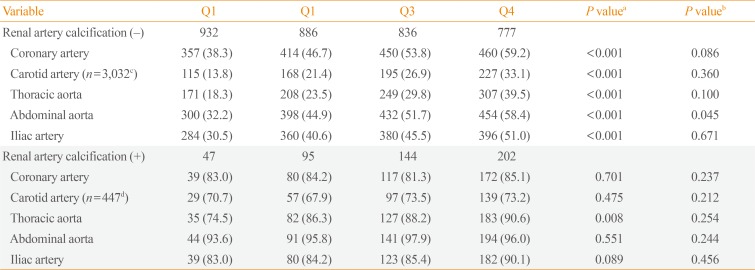




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download