Abstract
BACKGROUND/OBJECTIVES
MATERIALS/METHODS
RESULTS
CONCLUSIONS
Figures and Tables
 | Fig. 1Size Exclusion Chromatography profiles.(A) CLE and (B) artificial CLE digest and (C) molecular weight standards were detected at 214 nm. Elution time of standards were the following: a - Aprotinin (MW: 6,512, elution time: 22.314 min); b - angiotensin II (MW: 1,046, elution time: 32.504 min); c - triglycine (MW: 189, elution time: 33.664); and d - glycine (MW: 75, elution time: 35.529 min).
|
 | Fig. 2Acid mucopolysaccharide production by artificial CLE digest in ATDC5 cells.Artificial CLE digest was added at concentrations of 100, 500, 1,000 and 2,000 µg/mL. Values represent mean ± SD. Alcian blue-stained area is expressed as a relative value with control as 100%. **P < 0.01 compared with control, n = 3.
|
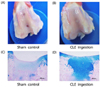 | Fig. 3Anatomy of the knee joint of the animals after 3 weeks CLE administration.(A) Macroscopic observation at burr holes in the sham control. (B) Macroscopic observation at burr holes in the CLE ingestion group. (C) Alcian blue staining of the burr holes in the sham control. (D) Alcian blue staining of the burr holes in the CLE ingestion group.
|
 | Fig. 4Mass spectrometry chromatograms of artificial CLE digest in SEC fractions 36–39 ((A); m/z = 120–200, (B); m/z = 200–250, (C); m/z = 250–300, (D); m/z = 300–400, (E); m/z = 400–1,000).Peaks marked with Single-Letter Amino Acid Code and “Hyp” represent amino acids. Peaks marked with lowercase represent peptides. Peaks marked with asterisk are compounds that could not be estimated as peptides with any combination of amino acids.
|
 | Fig. 5Acid mucopolysaccharide production in ATDC5 cells cultured with CLE-derived peptides identified from human plasma.Each sample was added at a concentration of 500 µM. Values represent mean ± SD. Alcian blue-stained area is expressed as a relative value with control as 100%. * P < 0.05 compared with control, n = 3.
|
 | Fig. 6Acid mucopolysaccharide production by Phe-Hyp in ATDC5 cells.Phe-Hyp was added at concentrations of 12.5, 25, 50 and 75 µM. Values represent mean ± SD. Alcian blue-stained area is expressed as a relative value with control as 100%. * P < 0.05 and ** P < 0.01 compared with control, n = 3.
|
 | Fig. 7Comparison of acid mucopolysaccharide production in ATDC5 cells.(A) Comparison of artificial CLE digest (2,000 µg/mL) and Phe-Hyp (21 µg/mL) in consideration of material balance. * P < 0.05, n.s., not significant. (B) Comparison of Pro-Hyp (2.5 mM) and Phe-Hyp (50 µM) in consideration of the abundance ratio in human plasma. Values represent mean ± SD. Alcian blue-stained area is expressed as a relative value with control as 100%. * P < 0.05 compared with control, n = 3.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download