Abstract
Catabacter hongkongensis is an anaerobic gram-positive coccobacillus that was first isolated in Hong Kong. It is infectious and causes high mortality in patients with rare but underlying diseases. Alistipes indistinctus is an anaerobic gram-negative coccobacillus. This bacterium is a common member of the human intestinal microbiota. We report a case of C. hongkongensis and A. indistinctus isolated from blood cultures of a patient with acute appendicitis. A 35-year-old female patient with no specific medical history was admitted to the hospital due to abdominal pain, vomiting, nausea, and diarrhea experienced on the day before admission. On admission, laboratory tests revealed leukocytosis, neutropenia, and elevated C–reactive protein and procalcitonin levels. Following an abdominal computed tomography showing acute appendicitis with suspected perforation, emergency surgery was performed. Growth was observed in two anaerobic blood culture bottles after four days. After further culturing of the bacteria on Brucella Blood Agar, two types of bacteria were obtained. The two bacterial isolates, one gram-positive and one gram-negative, were unable to be identified using matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). Thus, 16S rRNA gene sequence analysis was performed, resulting in identification of the bacteria as C. hongkongensis and A. indistinctus. The patient was administered antibiotics and discharged two days after surgery. Although MALDI-TOF MS enables fast and accurate identification of bacteria, C. hongkongensis and A. indistinctus were not listed in the spectral library, and 16S rRNA gene sequence analysis was useful for identifying the two bacteria.
References
1. Lau SK, McNabb A, Woo GK, Hoang L, Fung AM, Chung LM, et al. Catabacter hongkongensis gen. nov., sp. nov., isolated from blood cultures of patients from Hong Kong and Canada. J Clin Microbiol. 2007; 45:395–401.
2. Lau SK, Fan RY, Lo HW, Ng RH, Wong SS, Li IW, et al. High mortality associated with Catabacter hongkongensis bacteremia. J Clin Microbiol. 2012; 50:2239–43.

3. Elsendoorn A, Robert R, Culos A, Roblot F, Burucoa C. Catabacter hongkongensis bacteremia with fatal septic shock. Emerg Infect Dis. 2011; 17:1330–1.

4. Smith K, Pandey SK, Ussher JE. Bacteraemia caused by Catabacter hongkongensis. Anaerobe. 2012; 18:366–8.

5. Nagai F, Morotomi M, Watanabe Y, Sakon H, Tanaka R. Alistipes indis-tinctus sp. nov. and Odoribacter laneus sp. nov., common members of the human intestinal microbiota isolated from faeces. Int J Syst Evol Microbiol. 2010; 60:1296–302.

6. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 27th ed.CLSI supplement, M100. Wayne PA: Clinical and Laboratory Standards Institute;2017.
7. Choi YJ, Won EJ, Kim SH, Shin MG, Shin JH, Suh SP. First case report of bacteremia due to Catabacter hongkongensis in a Korean patient. Ann Lab Med. 2017; 37:84–7.

8. Torri A, Delbianco F, Baccarini FD, Fusari M, Bertini S, Congestri F, et al. First report of sepsis due to Catabacter hongkongensis in an Italian patient. New Microbes New Infect. 2015; 9:54–5.

9. Petrov VA, Saltykova IV, Zhukova IA, Alifrova VM, Zhukova NG, Doro-feeva YB, et al. Analysis of gut microbiota in patients with Parkinson's disease. Bull Exp Biol Med. 2017; 162:734–7.

10. Woods CW, Bressler AM, LiPuma JJ, Alexander BD, Clements DA, Weber DJ, et al. Virulence associated with outbreak-related strains of Burkholderia cepacia complex among a cohort of patients with bacteremia. Clin Infect Dis. 2004; 38:1243–50.

11. Chun S, Yun JW, Huh HJ, Lee NY. Low virulence? Clinical characteristics of Raoultella planticola bacteremia. Infection. 2014; 42:899–904.

12. Shkoporov AN, Chaplin AV, Khokhlova EV, Shcherbakova VA, Motu-zova OV, Bozhenko VK, et al. Alistipes inops sp. nov. and Coprobac-ter secundus sp. nov., isolated from human faeces. Int J Syst Evol Microbiol. 2015; 65:4580–8.

13. Fenner L, Roux V, Ananian P, Raoult D. Alistipes finegoldii in blood cultures from colon cancer patients. Emerg Infect Dis. 2007; 13:1260–2.
14. Hecht DW. Prevalence of antibiotic resistance in anaerobic bacteria: worrisome developments. Clin Infect Dis. 2004; 39:92–7.

15. Bizzini A, Durussel C, Bille J, Greub G, Prod'hom G. Performance of matrix-assisted laser desorption ionization-time of fight mass spectrometry for identifcation of bacterial strains routinely isolated in a clinical microbiology laboratory. J Clin Microbiol. 2010; 48:1549–54.
Fig. 1.
Gram stain microscopy of isolates after 72 hours of anaerobic incubation on Brucella Blood Agar. (A) Microscopic examination (×1,000) of the isolate showed gram-positive coccobacilli (Catabacter hongkongensis). (B) Microscopic examination (×1,000) of the second isolate revealed gram-negative coccobacilli (Alistipes indistinctus).
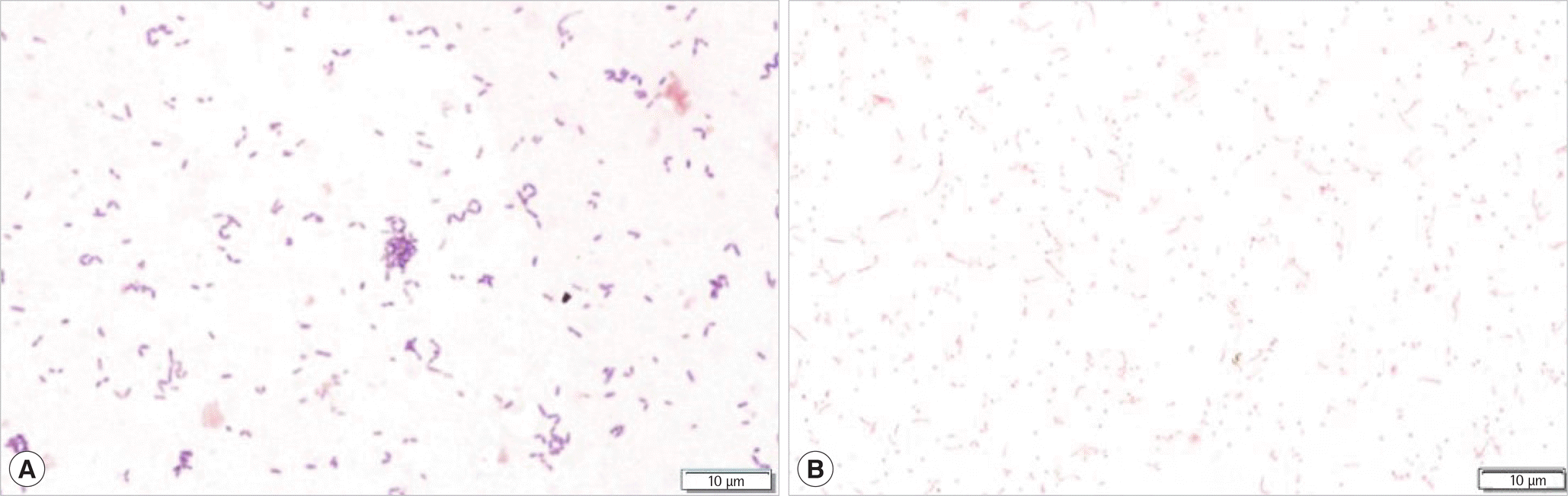
Table 1.
Minimal inhibitory concentration of antimicrobial agents against Catabacter hongkongensis and Alistipes indistinctus
| Antimicrobial agent (measurable range, μg/mL) | Catabacter hongkongensis | Alistipes indistinctus |
|---|---|---|
| CM (0.016–256) | >256 | >256 |
| TZP (0.016–256)∗ | 0.25 | >256 |
| IP (0.002–32) | 0.064 | 0.38 |
| VA (0.016–256) | 0.75 | >256 |
| TX (0.002–32) | 6 | >32 |
| MZ (0.016–256) | ≤0.016 | ≤0.016 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download