Abstract
Purpose:
This study examined the antioxidant and cancer cell growth inhibitory activities of an ethanol extract and different solvent fractions of Mesembryanthemum crystallinum L. (ice plant).
Methods:
The ice plant was freeze-dried, extracted with 99.9% ethanol, and then fractionated with hexane, ethyl acetate, butanol, and water. The total polyphenol content (TPC), total carotenoid content (TCC), 2,2-diphenyl-1-picrylhydrazyl radical-scavenging activity (RSA), and ferric reducing antioxidant power (FRAP) were measured. Assays using 2',7'-dichlorofluorescin-diacetate and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide were performed to measure the intracellular reactive oxygen species (ROS) and cell growth, respectively. Annexin V/propidium iodide staining and cell cycle analysis were performed for the detection of apoptosis and cell cycle arrest.
Results:
TPC, TCC, RSA, and FRAP of the ethanol extract (EE) were 3.7 mg gallic acid equivalent/g, 13.2 μg/g, 21.0% (at a concentration of 5 mg/mL), and 21.0% (at a concentration of 5 mg/mL), respectively. Among the different solvent fractions, the butanol fraction (BF) showed the highest TPC (5.4 mg gallic acid equivalent/g), TCC (86.6 μg/g), RSA (34.9% at 5 mg/mL), and FRAP (80.8% at 5 mg/mL). Treatment of HCT116 human colon cancer cells with EE and BF at concentrations of 250 and 500 μg/mL reduced the levels of intracellular ROS. Concomitantly, EE and BF resulted in the dose-dependent inhibition of cell growth (at the concentrations of 125, 250, and 500 μg/mL for 24~48 h) and the induction of apoptosis (at the concentrations of 250 and 500 μg/mL for 48 h) in HCT116 cells. An increased G2/M cell population was also found in the BF-treated cells.
Go to : 
REFERENCES
1.Agarie S., Kawaguchi A., Kodera A., Sunagawa H., Kojima H., Nose A, et al. Potential of the common ice plant, Mesembryanthemum crystallinum as a new high-functional food as evaluated by polyol accumulation. Plant Prod Sci. 2009. 12(1):37–46.
2.Valko M., Leibfritz D., Moncol J., Cronin MT., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007. 39(1):44–84.

3.Kleinsmith LJ. Principles of cancer biology. San Francisco: Pearson Benjamin Cummings;2006.
4.Statistics Korea. Causes of death statistics in 2011. Daejeon: Statistics Korea;2012.
5.Jung KW., Won YJ., Oh CM., Kong HJ., Lee DH., Lee KH. Prediction of cancer incidence and mortality in Korea, 2017. Cancer Res Treat. 2017. 49(2):306–312.

6.Boyle P., Levin B. World cancer report 2008. Geneva: International Agency for Research on Cancer;2008.
8.Kang S., Kim S., Ha S., Lee C., Nam S. Biochemical components and physiological activities of ice plant (Mesembryanthemum crystallinum). J Korean Soc Food Sci Nutr. 2016. 45(12):1732–1739.

9.Amari T., Ghnaya T., Debez A., Taamali M., Ben Youssef N., Lucchini G, et al. Comparative Ni tolerance and accumulation potentials between Mesembryanthemum crystallinum (halophyte) and Brassica juncea: metal accumulation, nutrient status and photosynthetic activity. J Plant Physiol. 2014. 171(17):1634–1644.

10.Nam S., Kang S., Kim S., Ko K. Effect of fermented ice plant (Mesembryanthemum crystallinum L.) extracts against antioxidant, antidiabetic and liver protection. J Life Sci. 2017. 27(8):909–918.
11.Lee SY., Choi HD., Yu SN., Kim SH., Park SK., Ahn SC. Biological activities of Mesembryanthemum crystallinum (ice plant) extract. J Life Sci. 2015. 25(6):638–645.

12.Ibtissem B., Abdelly C., Sfar S. Antioxidant and antibacterial properties of Mesembryanthemum crystallinum and Carpobrotus edulis extracts. Adv Chem Eng Sci. 2012. 2(3):359–365.
13.Kang SM., Kim SJ., Nam S. Inhibitory effect of cell differentiation against 3T3-L1 pre-adipocytes and angiotensin converting enzyme (ACE) activity of ice plant (Mesembryanthemum crystallinum). J Korean Soc Food Sci Nutr. 2017. 46(8):1012–1017.
14.Lee BH., Lee CC., Wu SC. Ice plant (Mesembryanthemum crystallinum) improves hyperglycaemia and memory impairments in a Wistar rat model of streptozotocin-induced diabetes. J Sci Food Agric. 2014. 94(11):2266–2273.

15.Bates SH., Jones RB., Bailey CJ. Insulin-like effect of pinitol. Br J Pharmacol. 2000. 130(8):1944–1948.

16.Singleton VL., Rossi JA Jr. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am J Enol Vitic. 1965. 16(3):144–158.
17.Wellburn AR. The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol. 1994. 144(3):307–313.
18.Brand-Williams W., Cuvelier ME., Berset C. Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss Technol. 1995. 28(1):25–30.

19.Benzie IF., Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996. 239(1):70–76.

20.Wang H., Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999. 27(5-6):612–616.
21.Kwak Y., Ju J. Inhibitory activities of Perilla frutescens britton leaf extract against the growth, migration, and adhesion of human cancer cells. Nutr Res Pract. 2015. 9(1):11–16.
22.Bundscherer A., Malsy M., Lange R., Hofmann P., Metterlein T., Graf BM, et al. Cell harvesting method influences results of apoptosis analysis by annexin V staining. Anticancer Res. 2013. 33(8):3201–3204.
23.Irons R., Tsuji PA., Carlson BA., Ouyang P., Yoo MH., Xu XM, et al. Deficiency in the 15-kDa selenoprotein inhibits tumorigenicity and metastasis of colon cancer cells. Cancer Prev Res (Phila). 2010. 3(5):630–639.

24.National Academy of agricultural Science. Tables of food functional composition. Suwon: Rural Development Administration;2009.
25.Klaunig JE., Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004. 44(1):239–267.

27.Weinberg RA. The biology of cancer. New York (NY): Garland Science;2007.
28.Kwak Y., Ki S., Noh EK., Shin HN., Han YJ., Lee Y, et al. Comparison of antioxidant and anti-proliferative activities of perilla (Perilla frutescens Britton) and sesame (Seasamum indicum L.) leaf extracts. Korean J Food Cookery Sci. 2013. 29(3):241–248.

29.Woo WS. Phenolic compound. In Natural product chemistry method. 2nd ed.Seoul: Seoul National University;1995.
30.Seo Y., Kim H. Antioxidant activity of fruits of Ligustrum japonicum. Ocean Polar Res. 2017. 39(2):115–124.

31.Jeon SM., Lee JY., Kim HW., Lee YM., Jang HH., Hwang KA, et al. Antioxidant activity of extracts and fractions from Aster scaber. J Korean Soc Food Sci Nutr. 2012. 41(9):1197–1204.

32.Pak WM., Kim KBWR., Kim MJ., Kang BK., Bark SW., Kim BR, et al. Antioxidative effect of extracts from different parts of Kohlrabi. J Appl Biol Chem. 2014. 57(4):353–358.

33.Kim SM., Kim DY., Park HR., Seo JH., Yeom BY., Jin YJ, et al. Screening the antioxidant components and antioxidant activity of extracts derived from five varieties of edible spring flowers. Korean J Food Sci Technol. 2014. 46(1):13–18.
34.Lee JE., Kim JH., Kim MY. Changes in phenolic composition, antioxidant and antidiabetic properties of Jeju Citrus sudachi as influenced by maturity. J Life Sci. 2015. 25(11):1311–1318.

35.Prasad S., Gupta SC., Tyagi AK. Reactive oxygen species (ROS) and cancer: role of antioxidative nutraceuticals. Cancer Lett. 2017. 387:95–105.

36.Kang SM., Hong JG. Antioxidant activities, production of reactive oxygen species, and cytotoxic properties of fractions from aerial parts of glasswort (Salicornia herbacea L.). Korean J Food Sci Technol. 2016. 48(6):574–581.

37.Lee MJ., Lee SE., Choi NR., Jo SH., Cho S. Effects of hexane fraction of Dracocephalum palmatum Stephan leaf on human-derived prostate cancer cell death. Korean J Herbol. 2018. 33(4):69–76.
Go to : 
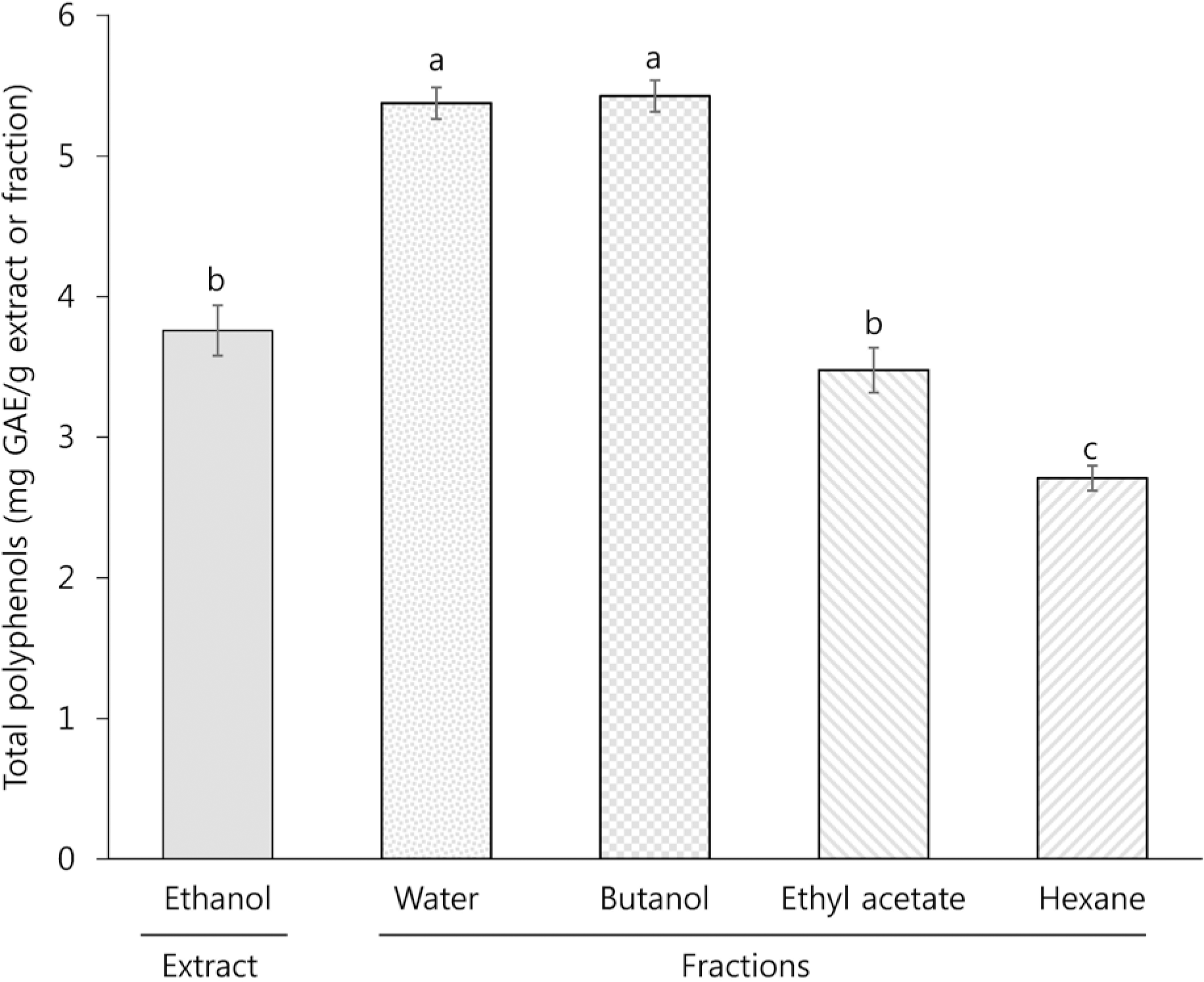 | Fig. 1Total polyphenol levels of ethanol extract and different solvent fraction prepared from Mesembryanthemum crystallinum L. GAE: gallic acid equivalent. All values are presented as mean ± SEM of ≥ 3 determinations. Different letters (a-c) mean significant difference among different samples by Duncan's multiple range test (p < 0.05). |
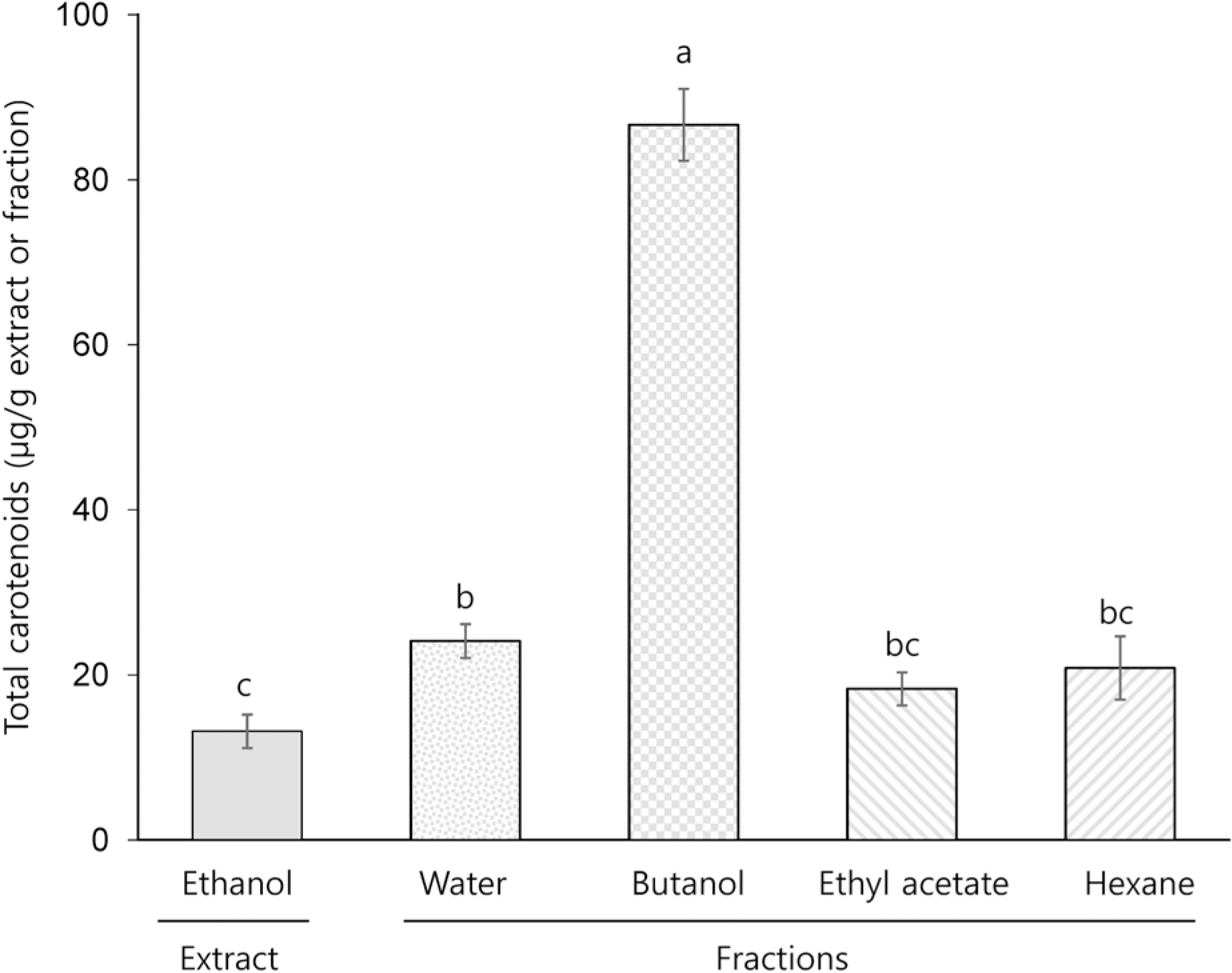 | Fig. 2Total carotenoid levels of ethanol extract and different solvent fraction prepared from Mesembryanthemum crystallinum L. All values are presented as mean ± SEM of ≥ 3 determinations. Different letters (a-c) mean significant difference among different samples by Duncan's multiple range test (p < 0.05). |
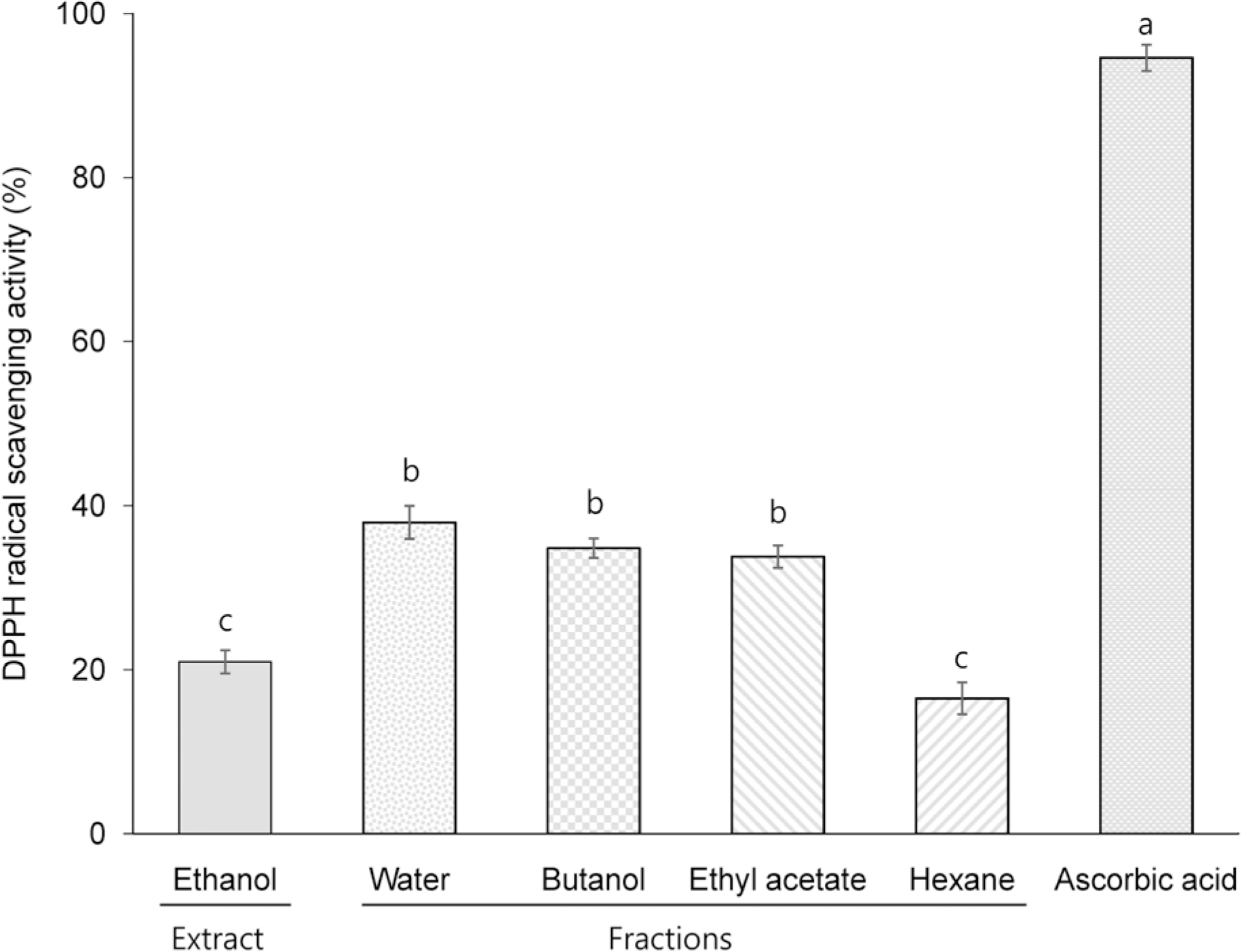 | Fig. 3DPPH radical scavenging activity of ethanol extract and different solvent fraction prepared from Mesembryanthemum crystallinum L. All values are presented as % of the control in the mean ± SEM of 3 determinations. Different letters (a-c) mean significant difference among different samples by Duncan's multiple range test (p < 0.05). |
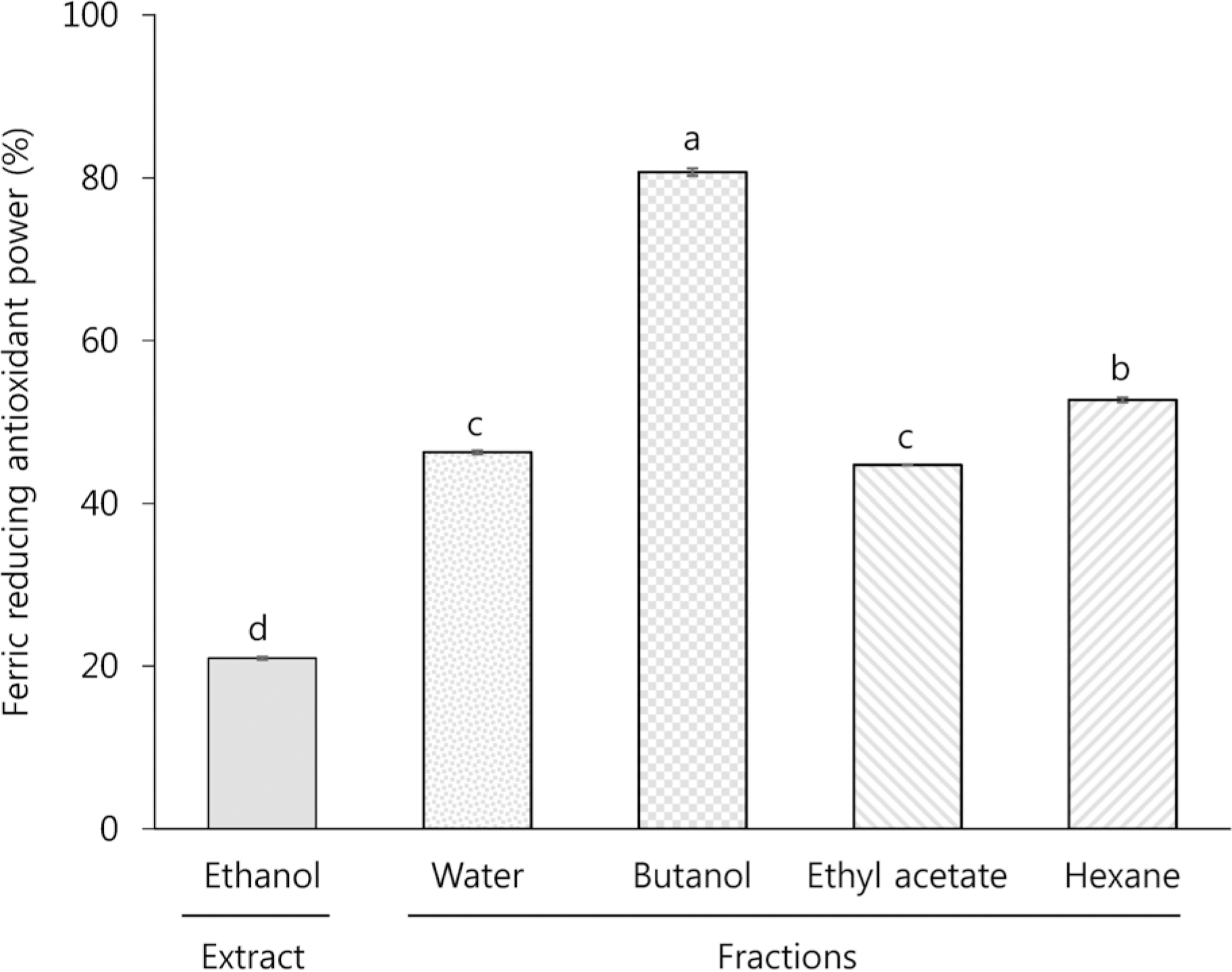 | Fig. 4Ferric reducing antioxidant power of ethanol extract and different solvent fraction prepared from Mesembryanthemum crystallinum L. All values are presented as % of the control in the mean ± SEM of 3 determinations. Different letters (a-d) mean significant difference among different samples by Duncan's multiple range test (p < 0.05). |
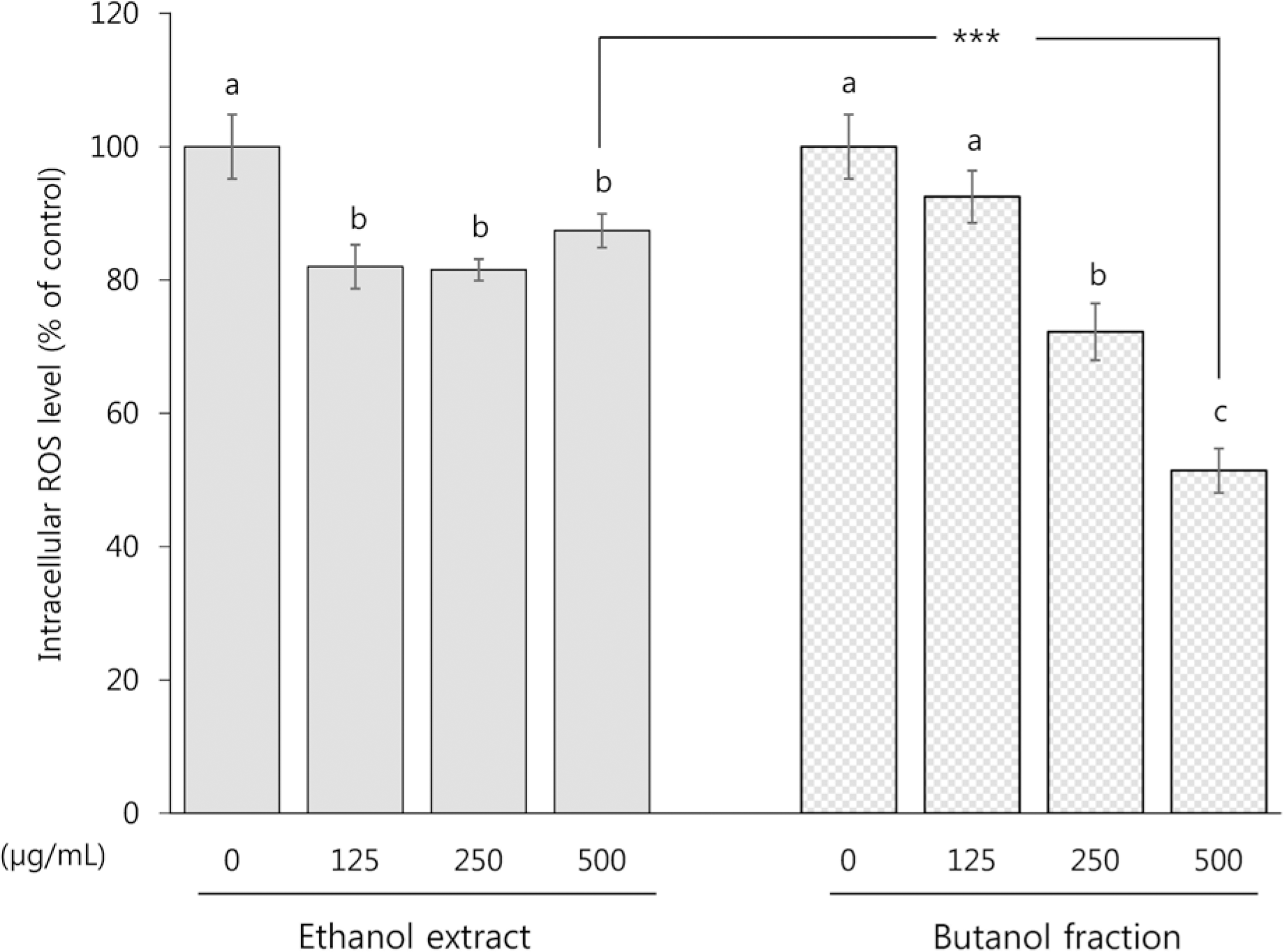 | Fig. 5Intracellular ROS scavenging activity of ethanol extract and butanol fraction prepared from Mesembryanthemum crystallinum L. in HCT116 colon cancer cells. HCT116 cells were treated with ethanol extract and butanol fraction at the concentrations of 125, 250, and 500 μg/mL for 48 h. All values are presented as mean ± SEM of ≥ 3 determinations. Different letters (a-c) mean significant difference among different concentrations by Duncan's multiple range test (p < 0.05). Asterisks mean statistical difference between two different sample at a respective concentration by two-tailed student t-test (∗∗∗ p < 0.001). |
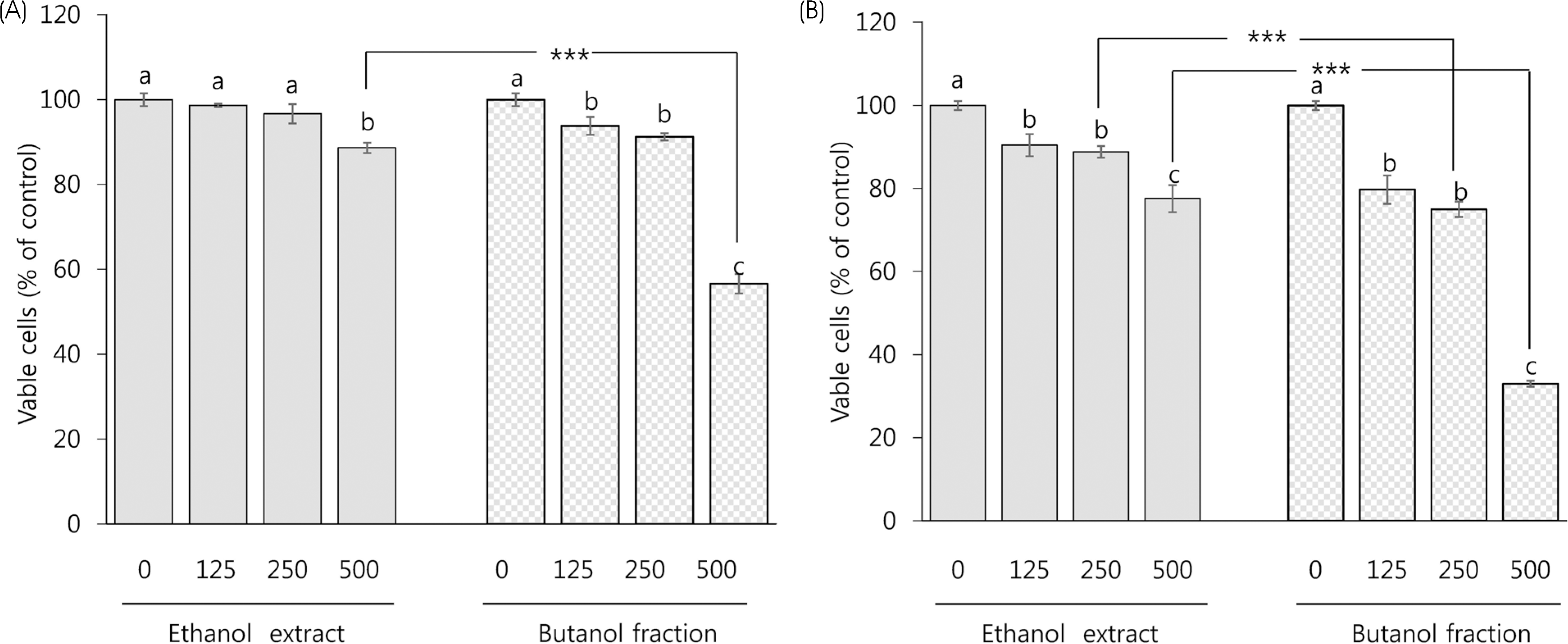 | Fig. 6Growth inhibitory activities of ethanol extract and butanol fraction prepared from Mesembryanthemum crystallinum L. in HCT116 colon cancer cells. HCT116 cells were treated with ethanol extract and butanol fraction at the concentrations of 125, 250, and 500 μg/mL for 24 h (A) and 48 h (B). All values are presented as mean ± SEM of ≥ 3 determinations. Different letters (a-c) mean significant difference among different concentrations by Duncan's multiple range test (p < 0.05). Asterisks mean statistical difference between two different sample at a respective concentration by two-tailed student t-test (∗∗∗ p < 0.001). |
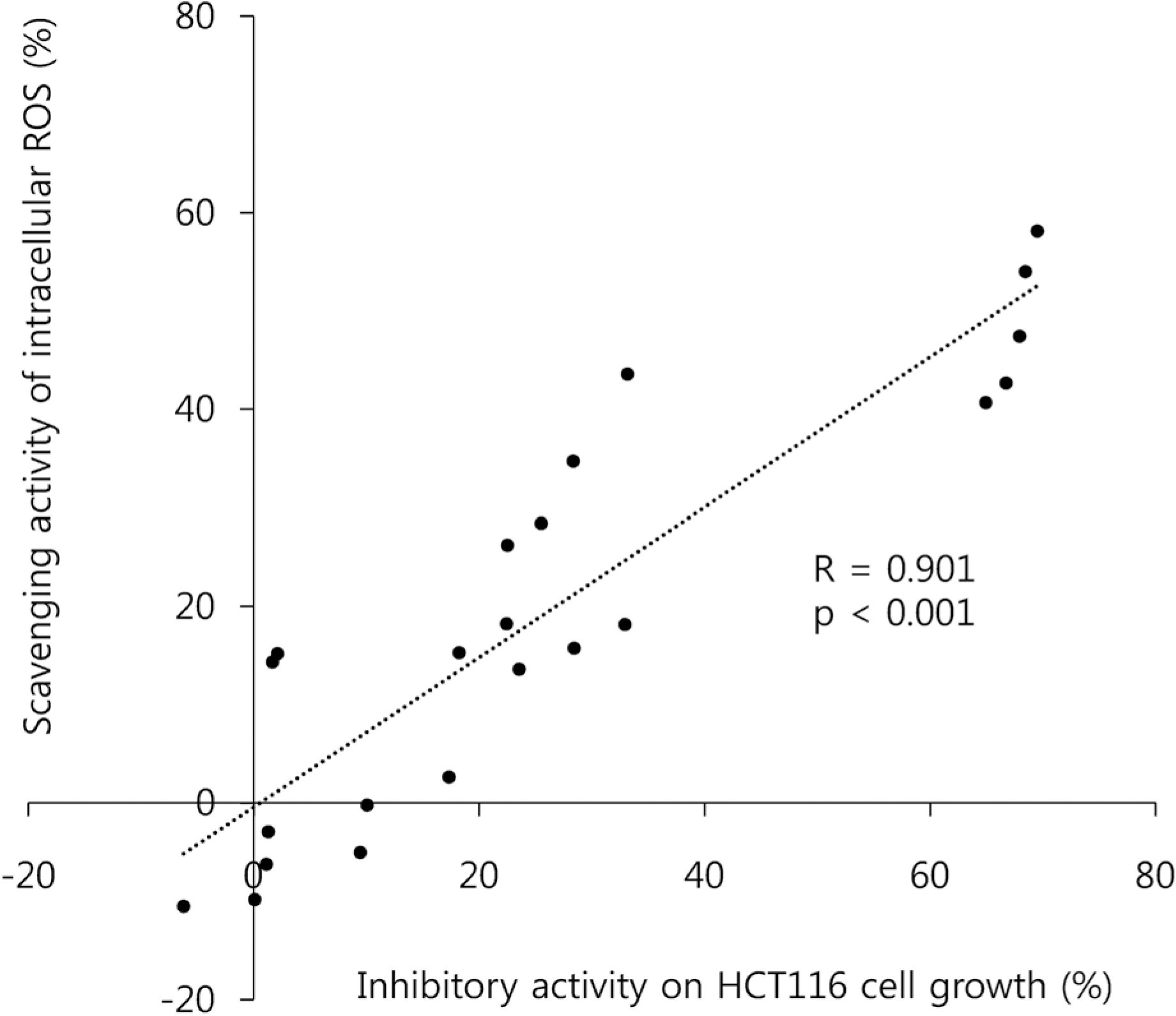 | Fig. 7Correlation between intracellular ROS scavenging activity and growth inhibitory activities of the butanol fraction prepared from Mesembryanthemum crystallinum L. in HCT116 colon cancer cells. Pearson's correlation analysis was performed. |
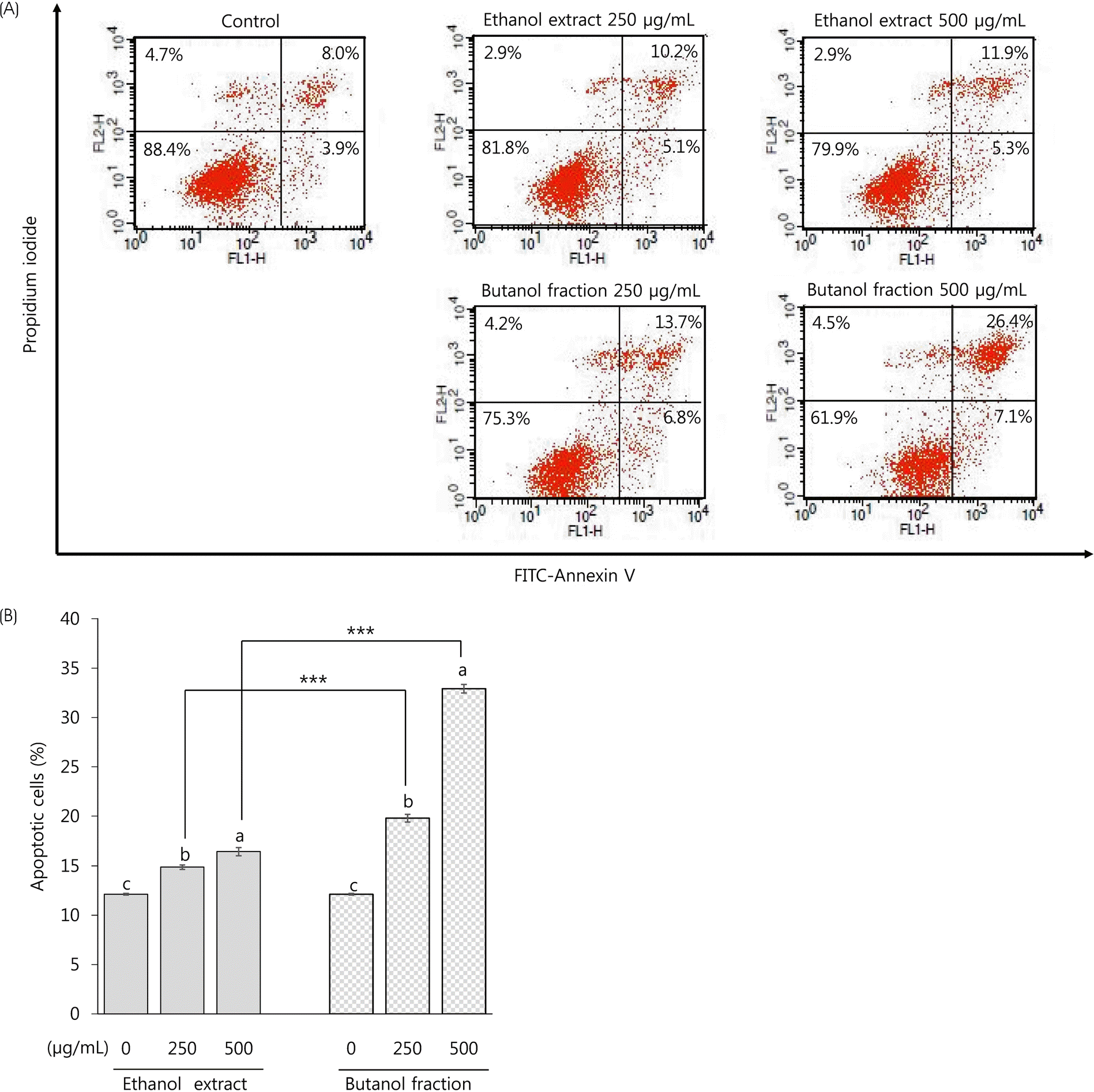 | Fig. 8Apoptosis-inducing activity of ethanol extract and butanol fraction prepared from Mesembryanthemum crystallinum L. in HCT116 colon cancer cells. HCT116 cells were treated with ethanol extract and butanol fraction at the concentrations of 250 and 500 μg/mL for 48 h. Representative histogram (A) and bar chart (B) of annexin/PI double staining assay. All values are presented as mean ± SEM of ≥ 3 determinations. Different letters (a-c) mean significant difference among different concentrations at a respective sample by Duncan's multiple range test (p < 0.05). Asterisks mean statistical difference between two different samples at a respective concentration by two-tailed student t-test (∗∗∗ p < 0.001). |
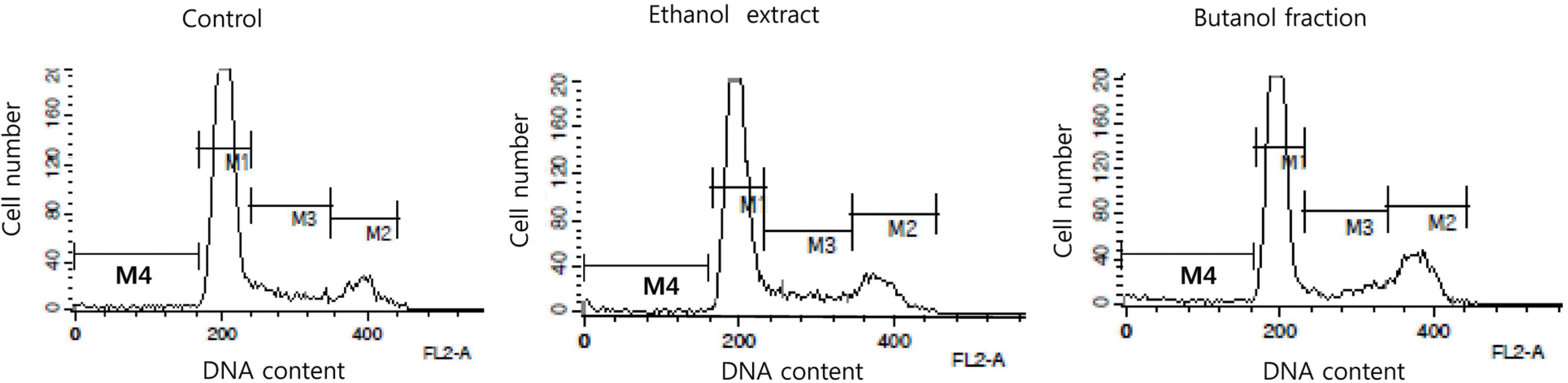 | Fig. 9Representative cell cycle of HCT116 colon cancer cells after the treatments of ethanol extract and butanol fraction prepared from Mesembryanthemum crystallinum L. HCT116 cells (3 × 105 cells/well) were treated with ethanol extract or butanol fraction at the concentrations of 500 μg/mL for 48h. M1: G0/G1 phase; M2: G2/M phase; M3: S phase; M4: sub-G1 phase. |
Table 1.
Cell cycle distribution of HCT116 colon cancer cells after the treatment of ethanol extract and butanol fraction prepared from Mesembryanthemum crystallinum L.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download