Abstract
Purpose:
The skin pH is maintained by epidermal lactate, free fatty acids (FFAs), and free amino acids (FAAs). As a significant determinant of skin health, the skin pH is increased (less acidic) under abnormal and aged skin conditions. In a search for dietary alternatives that would promote an acidic skin pH, this study investigated the dietary effects of Lactobacillus plantarum CJLP55 isolated from Korean kimchi on the skin pH, and epidermal levels of lactate, FFAs, and FAAs in adult subjects.
Methods:
Seventy eight subjects (mean age 24.9 ± 0.5 years, range 19~37 years) were assigned randomly to ingest CJLP55, Lactobacillus strain from kimchi, (n=39, CJLP group) or placebo supplements (n=39, placebo group) for 12 weeks in a double-blind, placebo-controlled trial. Skin pH and epidermal levels of lactate, FFAs and FFAs were assessed at 0, 6 and 12 weeks.
Results:
Although significant decreases in skin pH were observed in both the CJLP and placebo groups at 6 weeks, the skin pH was decreased significantly only in the CJLP group at 12 weeks. In parallel, the epidermal level of lactate in the CJLP group was also increased by 25.6% at 12 weeks. On the other hand, the epidermal level of FAAs were not altered in the CJLP and placebo groups, but the epidermal level of total FFAs, including palmitic acid and stearic acid, was lower in the CJLP group than in the placebo group over 12 weeks. The changes in the other FFAs, such as palmitoleic acid and oleic acid, were similar in the CJLP and placebo groups over 12 weeks.
Go to : 
REFERENCES
1.Fluhr JW., Elias PM. Stratum corneum pH: formation and function of the ‘acid mantle'. Exogenous Dermatology. 2002. 1(4):163–175.

2.Schmid-Wendtner MH., Korting HC. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol. 2006. 19(6):296–302.

3.Thurmon FM., Ottenstein B. Studies on the chemistry of human perspiration with especial reference to its lactic acid content. J Invest Dermatol. 1952. 18(4):333–339.

4.Rawlings AV., Harding CR. Moisturization and skin barrier function. Dermatol Ther. 2004. 17(Suppl 1):43–48.

5.Garidel P., Fölting B., Schaller I., Kerth A. The microstructure of the stratum corneum lipid barrier: mid-infrared spectroscopic studies of hydrated ceramide: palmitic acid: cholesterol model systems. Biophys Chem. 2010. 150(1-3):144–156.
6.Goffin P., Lorquet F., Kleerebezem M., Hols P. Major role of NAD-dependent lactate dehydrogenases in aerobic lactate utilization in Lactobacillus plantarum during early stationary phase. J Bacteriol. 2004. 186(19):6661–6666.
7.Fluhr JW., Kao J., Jain M., Ahn SK., Feingold KR., Elias PM. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J Invest Dermatol. 2001. 117(1):44–51.

8.Suzuki N., Ishizaki J., Yokota Y., Higashino K., Ono T., Ikeda M, et al. Structures, enzymatic properties, and expression of novel human and mouse secretory phospholipase A2s. J Biol Chem. 2000. 275(8):5785–5793.
9.Matsui T., Miyamoto K., Kubo A., Kawasaki H., Ebihara T., Hata K, et al. SASPase regulates stratum corneum hydration through profilaggrin-to-filaggrin processing. EMBO Mol Med. 2011. 3(6):320–333.

10.Sandilands A., Sutherland C., Irvine AD., McLean WH. Filaggrin in the frontline: role in skin barrier function and disease. J Cell Sci. 2009. 122(Pt 9):1285–1294.

11.Eberlein-König B., Schäfer T., Huss-Marp J., Darsow U., Möhrenschlager M., Herbert O, et al. Skin surface pH, stratum corneum hydration, trans-epidermal water loss and skin roughness related to atopic eczema and skin dryness in a population of primary school children. Acta Derm Venereol. 2000. 80(3):188–191.
12.Choi EH., Man MQ., Xu P., Xin S., Liu Z., Crumrine DA, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol. 2007. 127(12):2847–2856.

13.Nakagawa N., Sakai S., Matsumoto M., Yamada K., Nagano M., Yuki T, et al. Relationship between NMF (lactate and potassium) content and the physical properties of the stratum corneum in healthy subjects. J Invest Dermatol. 2004. 122(3):755–763.

14.Béke G., Dajnoki Z., Kapitány A., Gáspár K., Medgyesi B., Póliska S, et al. Immunotopographical differences of human skin. Front Immunol. 2018. 9:424.

15.Bourrie BC., Willing BP., Cotter PD. The microbiota and health promoting characteristics of the fermented beverage kefir. Front Microbiol. 2016. 7:647.

16.Guéniche A., Philippe D., Bastien P., Blum S., Buyukpamukcu E., Castiel-Higounenc I. Probiotics for photoprotection. Dermatoendocrinol. 2009. 1(5):275–279.

17.Valdéz JC., Peral MC., Rachid M., Santana M., Perdigón G. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: the potential use of probiotics in wound treatment. Clin Microbiol Infect. 2005. 11(6):472–479.

18.Lee DE., Huh CS., Ra J., Choi ID., Jeong JW., Kim SH, et al. Clinical evidence of effects of Lactobacillus plantarum HY7714 on skin aging: a randomized, double blind, placebo-controlled study. J Microbiol Biotechnol. 2015. 25(12):2160–2168.

19.Kim H., Kim HR., Jeong BJ., Lee SS., Kim TR., Jeong JH, et al. Effects of oral intake of kimchi-derived Lactobacillus plantarum K8 lysates on skin moisturizing. J Microbiol Biotechnol. 2015. 25(1):74–80.

20.Giri SS., Sen SS., Saha S., Sukumaran V., Park SC. Use of a potential probiotic, Lactobacillus plantarum L7, for the preparation of a rice-based fermented beverage. Front Microbiol. 2018. 9:473.

21.Lew LC., Liong MT. Bioactives from probiotics for dermal health: functions and benefits. J Appl Microbiol. 2013. 114(5):1241–1253.

22.Šeme H., Gjuračić K., Kos B., Fujs Š., Štempelj M., Petković H, et al. Acid resistance and response to pH-induced stress in two Lactobacillus plantarum strains with probiotic potential. Benef Microbes. 2015. 6(3):369–379.

23.Lowry OH., Rosebrough NJ., Farr AL., Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951. 193(1):265–275.

24.Folch J., Lees M., Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957. 226(1):497–509.

25.Di Giorgio J. Determination of serum lactic dehydrogenase isoenzymes by use of the “Diagnostest” cellulose acetate electrophoresis system. Clin Chem. 1971. 17(4):326–331.

26.Imahashi D. Quantitation of LDH isoenzymes by the fluorimetric and colorimetric methods. Can J Med Technol. 1968. 30(6):235–248.
27.Uchida Y., Hara M., Nishio H., Sidransky E., Inoue S., Otsuka F, et al. Epidermal sphingomyelins are precursors for selected stratum corneum ceramides. J Lipid Res. 2000. 41(12):2071–2082.

28.Tang W., Ziboh VA. Reversal of epidermal hyperproliferation in essential fatty acid deficient guinea pigs is accompanied by rapid generation of inositol triphosphate. Arch Dermatol Res. 1988. 280(5):286–292.

29.Wesley NO., Maibach HI. Racial (ethnic) differences in skin properties: the objective data. Am J Clin Dermatol. 2003. 4(12):843–860.
30.Jacobi U., Gautier J., Sterry W., Lademann J. Gender-related differences in the physiology of the stratum corneum. Dermatology. 2005. 211(4):312–317.

31.Visscher MO., Chatterjee R., Munson KA., Pickens WL., Hoath SB. Changes in diapered and nondiapered infant skin over the first month of life. Pediatr Dermatol. 2000. 17(1):45–51.
32.Hoeger PH., Enzmann CC. Skin physiology of the neonate and young infant: a prospective study of functional skin parameters during early infancy. Pediatr Dermatol. 2002. 19(3):256–262.

33.Zlotogorski A. Distribution of skin surface pH on the forehead and cheek of adults. Arch Dermatol Res. 1987. 279(6):398–401.

34.Lee B., Kim J., Hwang J., Cho Y. Dietary effect of green tea extract on epidermal levels of skin pH related factors, lactate dehydrogenase protein expression and activity in UV-irradiated hairless mice. J Nutr Health. 2016. 49(2):63–71.

35.Sugawara T., Kikuchi K., Tagami H., Aiba S., Sakai S. Decreased lactate and potassium levels in natural moisturizing factor from the stratum corneum of mild atopic dermatitis patients are involved with the reduced hydration state. J Dermatol Sci. 2012. 66(2):154–159.

37.Akaza N., Akamatsu H., Numata S., Matsusue M., Mashima Y., Miyawaki M, et al. Fatty acid compositions of triglycerides and free fatty acids in sebum depend on amount of triglycerides, and do not differ in presence or absence of acne vulgaris. J Dermatol. 2014. 41(12):1069–1076.

38.Lewis C Jr., Schmitt M., Hershey FB. Heterogeneity of lactic dehydrogenase of human skin. J Invest Dermatol. 1967. 48(3):221–225.

39.Ronquist G., Andersson A., Bendsoe N., Falck B. Human epidermal energy metabolism is functionally anaerobic. Exp Dermatol. 2003. 12(5):572–579.

40.Boussouar F., Grataroli R., Ji J., Benahmed M. Tumor necrosis factor-alpha stimulates lactate dehydrogenase A expression in porcine cultured sertoli cells: mechanisms of action. Endocrinology. 1999. 140(7):3054–3062.
Go to : 
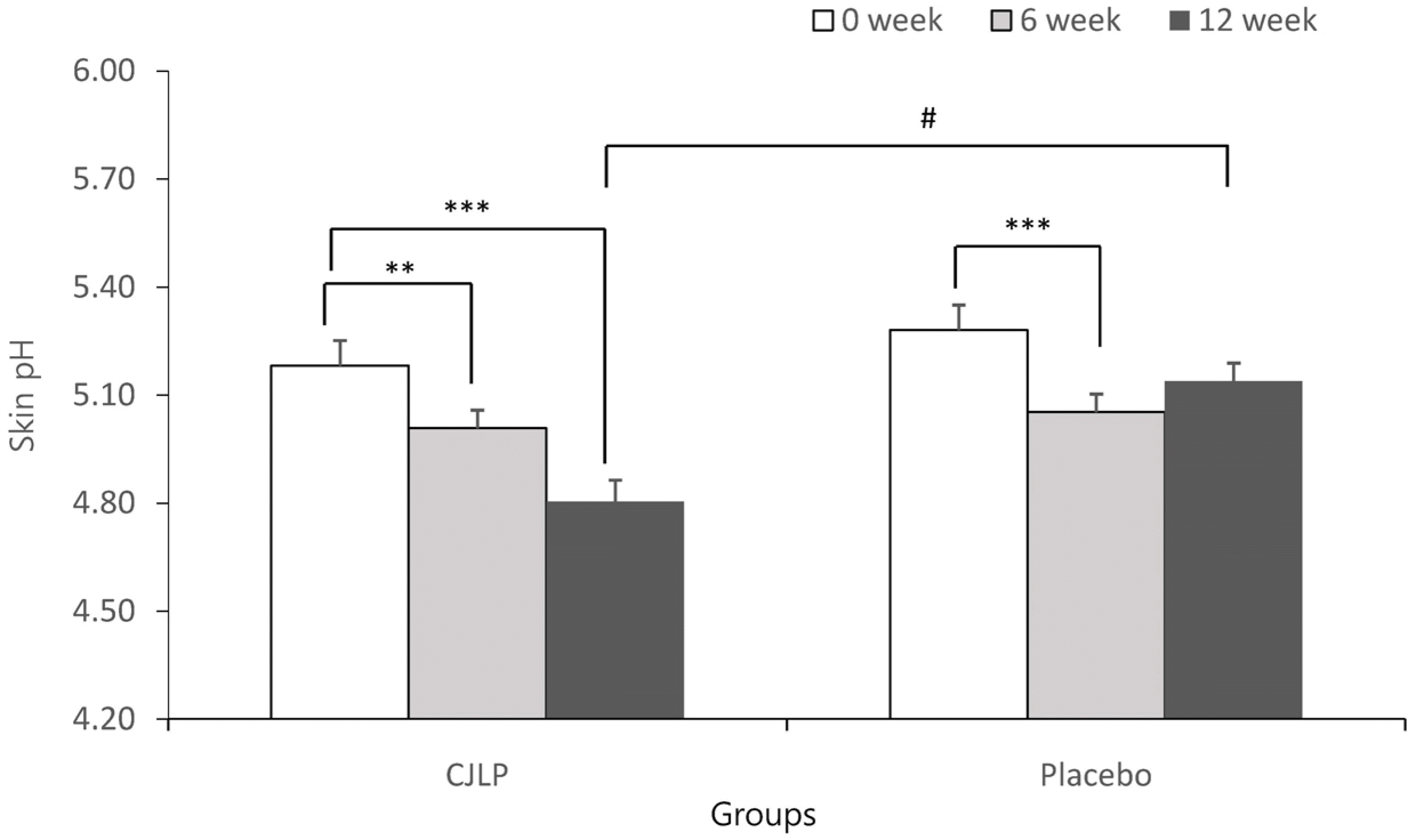 | Fig. 1Altered skin pH in groups. Skin surface pH was measured by skin pH meter. All values are means ± SEM (n = 39 / group). Differences from 0 week within CJLP or Placebo groups were evaluated by Student's paired t-test (∗ p < 0.05, ∗∗ p < 0.01, ∗∗∗ p < 0.001). Differences between CJLP and Placebo groups were evaluated by Student's unpaired t-test (# p < 0.05). |
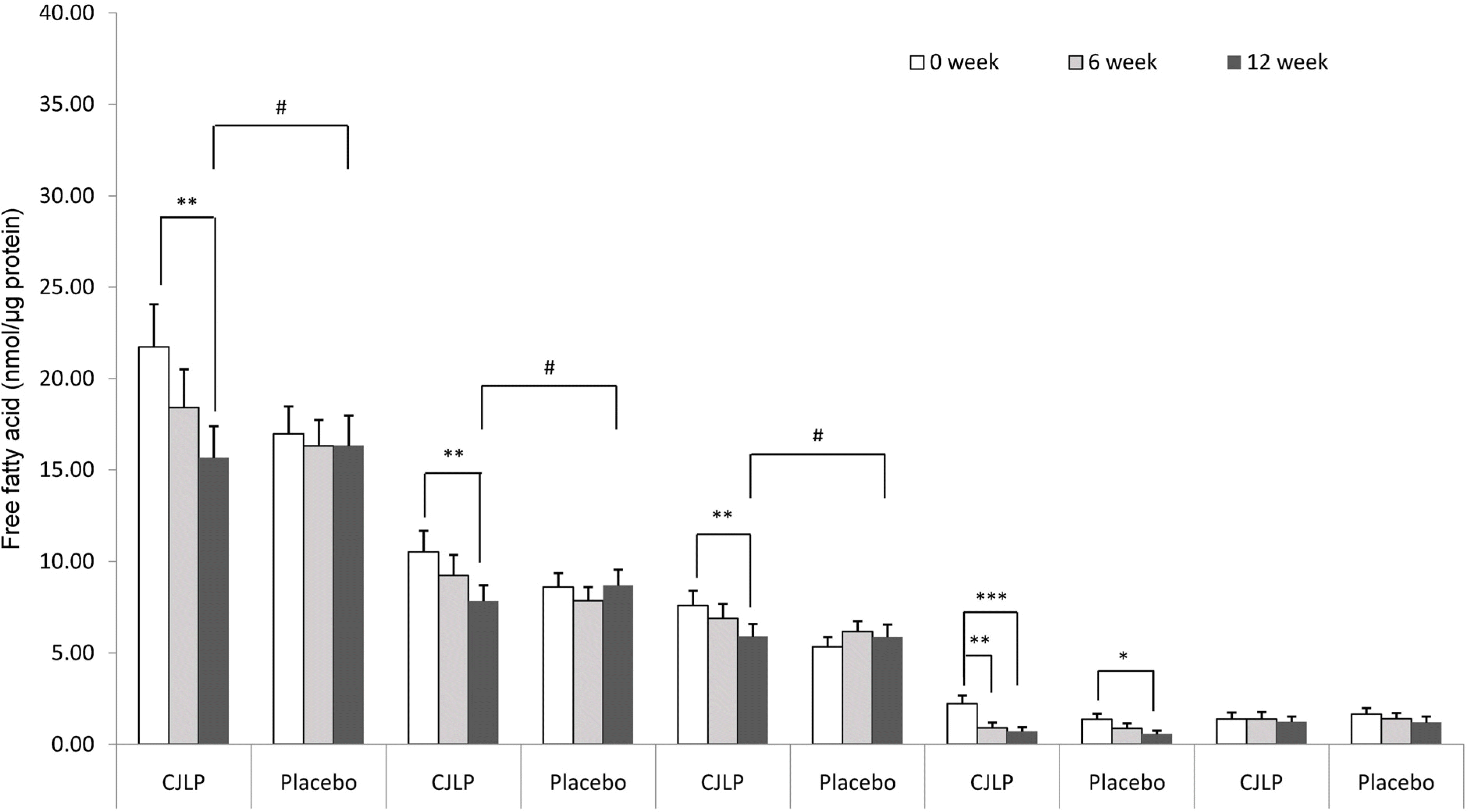 | Fig. 2Altered free fatty acid (FFA) levels in the skin surface of groups. Individual FFA was fractionated by high-performance thin-layer chromatography, eluted, and further analyzed by gas chromatography after acid methanolysis. All values are mean ± SEM (n = 39 / group). Differences from 0 week within CJLP or Placebo groups were evaluated by paired Student's t-test (∗ p < 0.05, ∗∗ p < 0.01, ∗∗∗ p < 0.001). Differences between CJLP and Placebo groups were evaluated by unpaired Student's t-test (# p < 0.05). |
Table 1.
Altered lactate levels in the skin surface of groups
Table 2.
Altered free amino levels in the skin surface of groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download