Abstract
Purpose:
Ulcerative colitis is a common inflammatory bowel disease. Prolonged colitis can be a risk factor for the development of colorectal cancer. Mulberry twig (MT, Sangzhi), a dry branch of Morus alba L., which is widely distributed throughout East Asia, has been shown to have anti-inflammatory activities in the cells. However, the effects of MT extracts on colitis in in vivo are limited. Therefore, in this study, we investigated the anti-inflammatory effects of MT extracts in the dextran sulfate sodium (DSS)-induced mouse colitis model.
Methods:
Six week-old, male ICR mice were divided into 3 groups: Control (n=5), DSS (n=7), and DSS+MT (n=7) groups. Mice in the DSS and DSS+MT groups were administrated 3% DSS in drinking water for 5 days to induce colitis. At the same time, water extracts of MT (5 g/kg body weight/day) were orally administered to mice in the DSS+MT groups for 5 days.
Results:
The MT extracts significantly reduced the clinical and pathological characteristics of colitis. Disease activity index, mucosal thickness, and colonocyte proliferation were significantly reduced in the DSS+MT group compared with the DSS group. Furthermore, MT administration reduced the levels of plasma TNF-α, IL-6, and the colonic myeloperoxidase activity as well as mRNA expression of TNF-α, IL-6, Cox-2, and iNOS.
Go to : 
REFERENCES
2.Eaden JA., Abrams KR., Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001. 48(4):526–535.

3.Gillen CD., Walmsley RS., Prior P., Andrews HA., Allan RN. Ulcerative colitis and Crohn's disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994. 35(11):1590–1592.

4.van Hogezand RA., Eichhorn RF., Choudry A., Veenendaal RA., Lamers CB. Malignancies in inflammatory bowel disease: fact or fiction? Scand J Gastroenterol Suppl. 2002. 37(236):48–53.
5.Yang SK., Loftus EV Jr., Sandborn WJ. Epidemiology of inflammatory bowel disease in Asia. Inflamm Bowel Dis. 2001. 7(3):260–270.

6.Ng WK., Wong SH., Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016. 14(2):111–119.

7.Yang DH., Yang SK. Trends in the incidence of ulcerative colitis in Korea. Korean J Med. 2009. 76(6):637–642.
8.Medzhitov R., Janeway CA Jr. Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997. 9(1):4–9.

10.Jang YJ., Leem HH., Jeon YH., Lee DH., Choi SW. Isolation and identification of α-glucosidase inhibitors from morus root bark. J Korean Soc Food Sci Nutr. 2015. 44(7):1090–1099.

11.Choi SW., Jang YJ., Lee YJ., Leem HH., Kim EO. Analysis of functional constituents in mulberry (Morus alba L.) twigs by different cultivars, producing areas, and heat processings. Prev Nutr Food Sci. 2013. 18(4):256–262.

12.Zhang Z., Shi L. Anti-inflammatory and analgesic properties of cis-mulberroside A from Ramulus mori. Fitoterapia. 2010. 81(3):214–218.

13.Chung KO., Kim BY., Lee MH., Kim YR., Chung HY., Park JH, et al. In-vitro and in-vivo anti-inflammatory effect of oxyresveratrol from Morus alba L. J Pharm Pharmacol. 2003. 55(12):1695–1700.

14.Cooper HS., Murthy SN., Shah RS., Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993. 69(2):238–249.
15.Hendrickson BA., Gokhale R., Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev. 2002. 15(1):79–94.

16.Kornbluth A., Sachar DB. Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010. 105(3):501–523.

17.Su C., Lichtenstein GR. Ulcerative colitis. Feldman M, Friedman LS, Brandt LJ, editors. editors.Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. Volume 2. 8th edition.Philadelphia (PA): Saunders;2006. p. 2499–2548.
18.Choi CH., Moon W., Kim YS., Kim ES., Lee BI., Jung Y, et al. Second Korean guideline for the management of ulcerative colitis. Korean J Gastroenterol. 2017. 69(1):1–28.

19.Okayasu I., Hatakeyama S., Yamada M., Ohkusa T., Inagaki Y., Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990. 98(3):694–702.

20.Walsh-Reitz MM., Huang EF., Musch MW., Chang EB., Martin TE., Kartha S, et al. AMP-18 protects barrier function of colonic epithelial cells: role of tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2005. 289(1):G163–G171.

21.Araki Y., Sugihara H., Hattori T. In vitro effects of dextran sulfate sodium on a Caco-2 cell line and plausible mechanisms for dextran sulfate sodium-induced colitis. Oncol Rep. 2006. 16(6):1357–1362.

22.Johansson ME., Gustafsson JK., Sjöberg KE., Petersson J., Holm L., Sjövall H, et al. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS One. 2010. 5(8):e12238.

23.Morgan ME., Zheng B., Koelink PJ., van de Kant HJ., Haazen LC., van Roest M, et al. New perspective on dextran sodium sulfate colitis: antigen-specific T cell development during intestinal inflammation. PLoS One. 2013. 8(7):e69936.

24.Forbes E., Murase T., Yang M., Matthaei KI., Lee JJ., Lee NA, et al. Immunopathogenesis of experimental ulcerative colitis is mediated by eosinophil peroxidase. J Immunol. 2004. 172(9):5664–5675.

25.Choi IY., Lee KT., Kim MC., Kim SJ., Kim DS., Jeon YD, et al. Anti-inflammatory effects of Cheongilppong on DSS-induced ulcerative colitis in mice. Orient Pharm Exp Med. 2011. 11(1):35–39.

26.Scott RJ., Hall PA., Haldane JS., van Noorden S., Price Y., Lane DP, et al. A comparison of immunohistochemical markers of cell proliferation with experimentally determined growth fraction. J Pathol. 1991. 165(2):173–178.

27.Min HY., Chung HJ., Kim EH., Kim S., Park EJ., Lee SK. Inhibition of cell growth and potentiation of tumor necrosis factor-α (TNF-α)-induced apoptosis by a phenanthroindoli-zidine alkaloid antofine in human colon cancer cells. Biochem Pharmacol. 2010. 80(9):1356–1364.

28.Hoffmann A., Leung TH., Baltimore D. Genetic analysis of NF-κ B/Rel transcription factors defines functional specificities. EMBO J. 2003. 22(20):5530–5539.
29.Kinoshita T., Ito H., Miki C. Serum interleukin-6 level reflects the tumor proliferative activity in patients with colorectal carcinoma. Cancer. 1999. 85(12):2526–2531.

30.Janssen-Heininger YM., Poynter ME., Baeuerle PA. Recent advances towards understanding redox mechanisms in the activation of nuclear factor κB. Free Radic Biol Med. 2000. 28(9):1317–1327.
31.Schulze-Osthoff K., Ferrari D., Riehemann K., Wesselborg S. Regulation of NF-κ B activation by MAP kinase cascades. Immunobiology. 1997. 198(1-3):35–49.
Go to : 
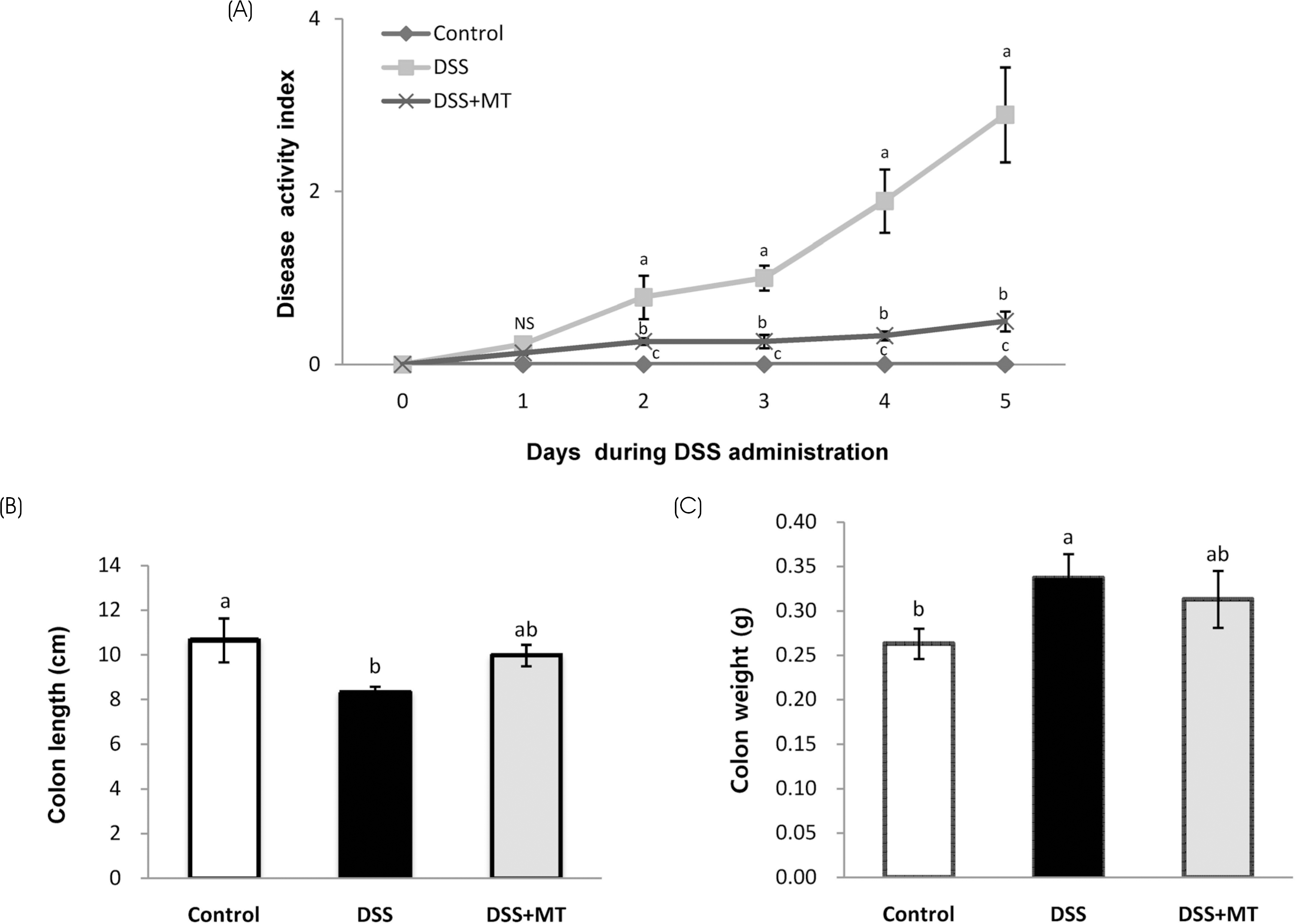 | Fig. 1Effects of MT on disease activity index, colon length and colon weight. (A) Disease activity index was measured daily during DSS administration. Large intestine was obtained after the DSS administration and their lengths (B) and weight (C) were measured. Value are presented as the mean ± SE. Means with different letters are significantly different at p〈 0.05 by Duncan's multiple range test. Control, group received water without DSS; DSS, group received 3% DSS in drinking water for 5 days; DSS+MT, group received 3% DSS and oral administration with MT water extracts (5 g/kg/day) for 5 days; DSS, dextran sulfate sodium; MT, mulberry twig; NS, not significant |
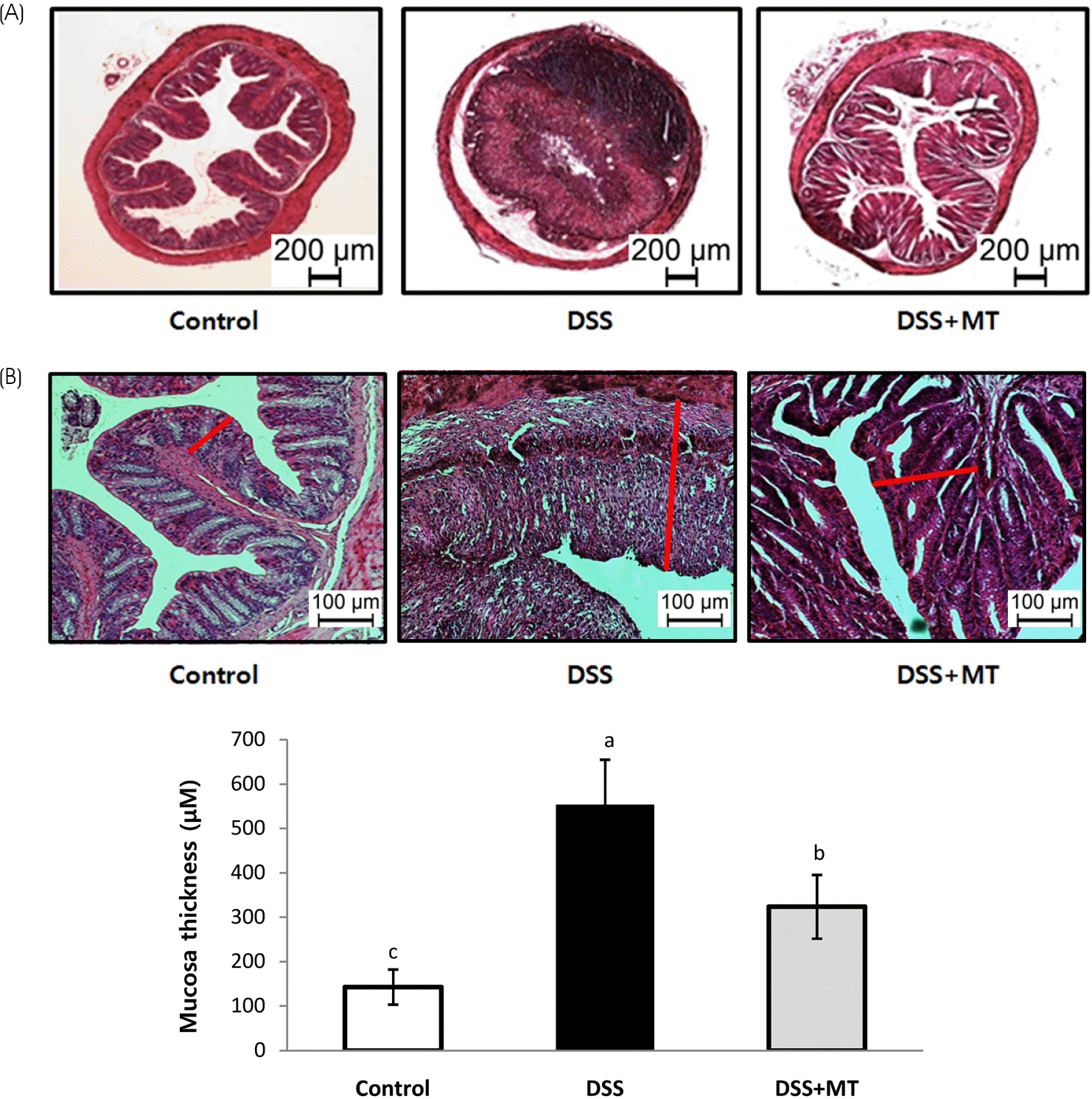 | Fig. 2Histopathological change of large intestine. The rectum of mouse was stained with hematoxylin and eosin. (A) Representative histological images of Control, DSS, and DSS+MT groups are shown. (B) Mucosal thickness was measured microscopically. Value are presented as the mean ± SE. Means with different letters are significantly different at p < 0.05 by Duncan's multiple range test. Control, group received water without DSS; DSS, group received 3% DSS in drinking water for 5 days; DSS+MT, group received 3% DSS and oral administration with MT water extracts (5 g/kg/day) for 5 days; DSS, dextran sulfate sodium; MT, mulberry twig |
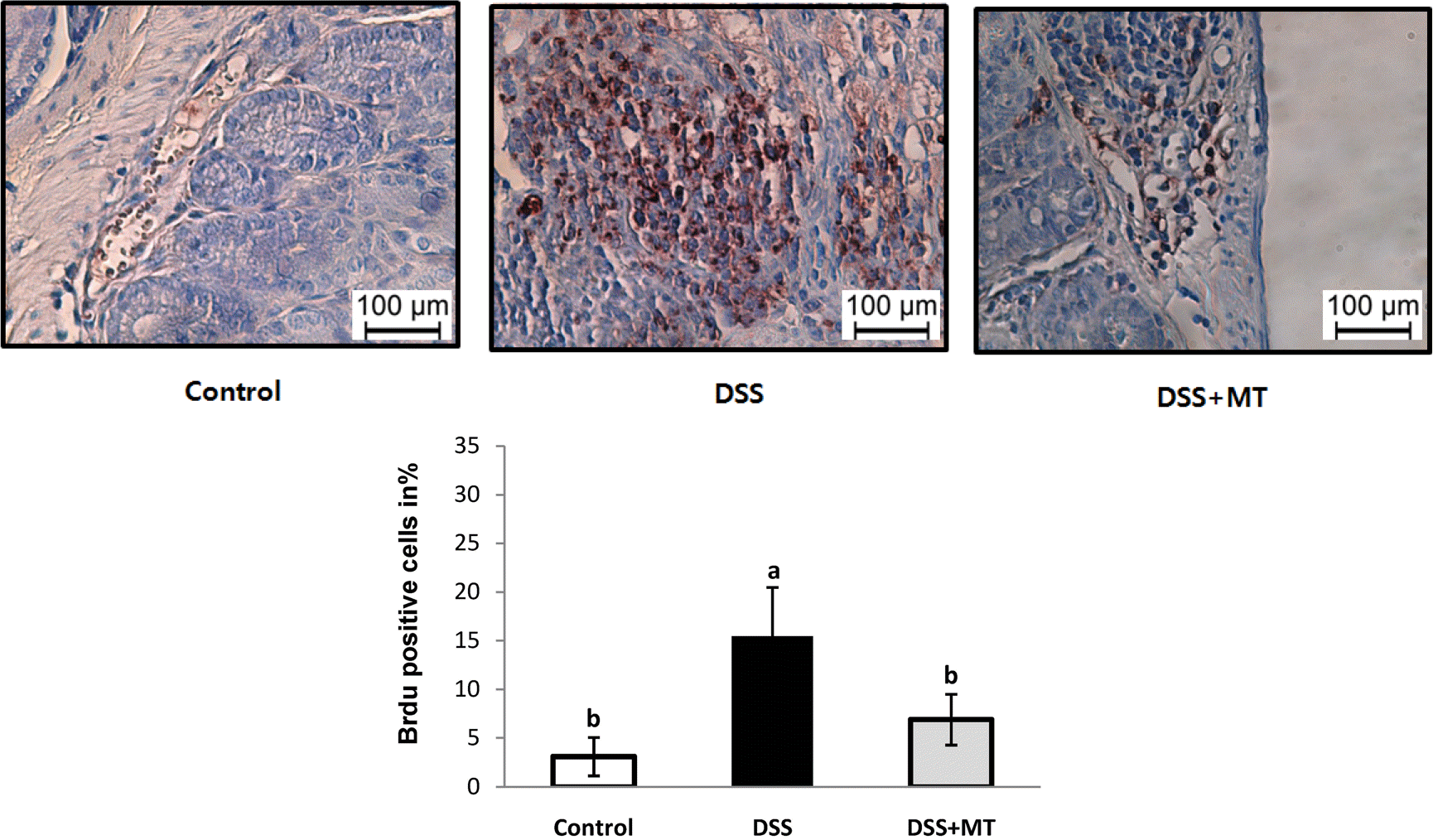 | Fig. 3Proliferation rate of colonocytes. BrdU incorporation was demonstrated immunohistochemically after i.p. injection of BrdU. The number of BrdU-labeled cells were counted and labeling index of BrdU was determined (BrdU-labeled cells/300 cells). Value are presented as the mean ± SE. Means with different letters are significantly different at p〈 0.05 by Duncan's multiple range test. Control, group received water without DSS; DSS, group received 3% DSS in drinking water for 5 days; DSS+MT, group received 3% DSS and oral administration with MT water extracts (5 g/kg/day) for 5 days; DSS, dextran sulfate sodium; MT, mulberry twig |
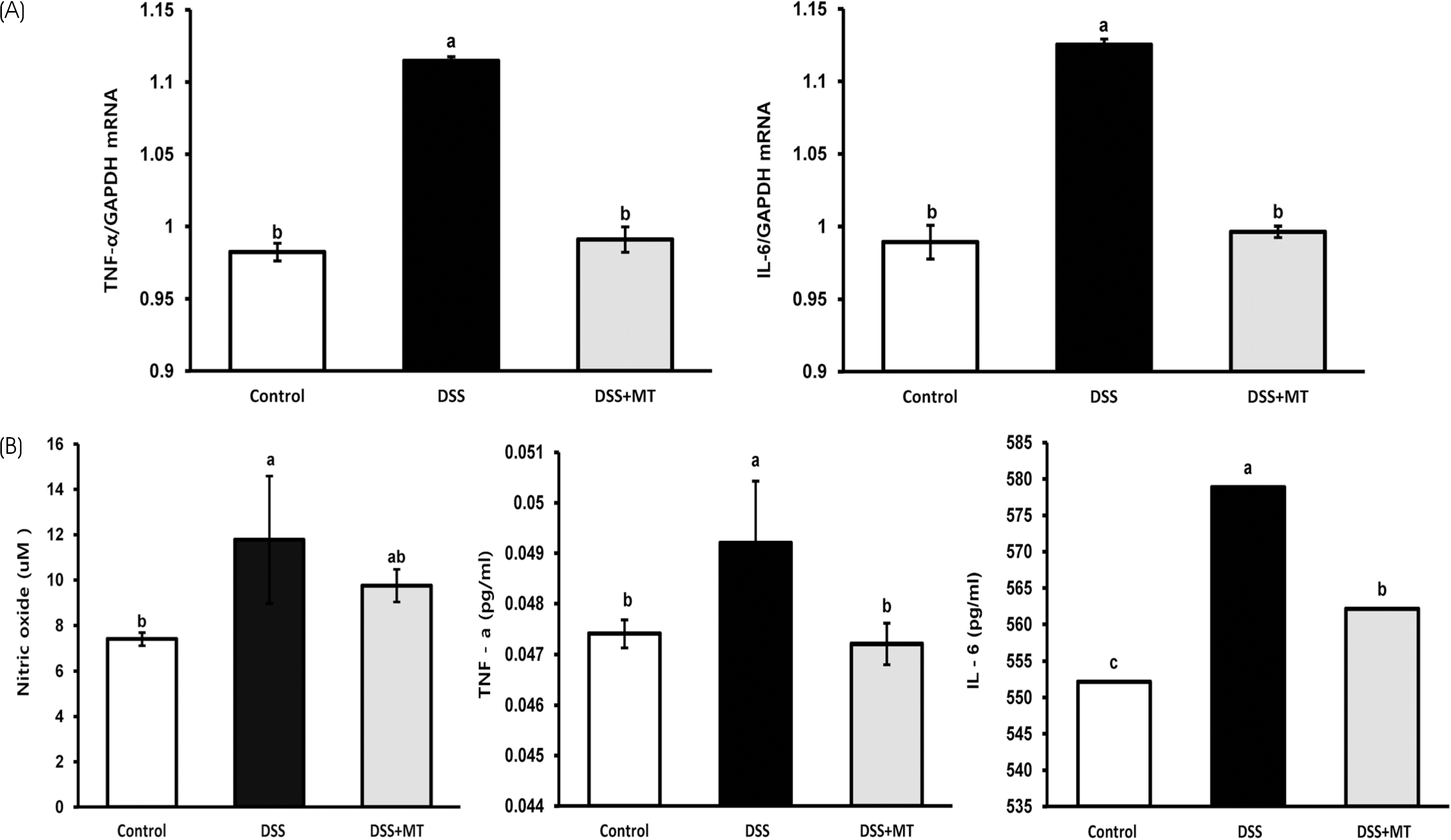 | Fig. 4The levels of inflammatory mediators in colorectal tissue and in plasma. (A) TNF-α and IL-6 mRNA expression in colorectal region were measured by qRT-PCR. (B) The levels of plasma NO, TNF-α and IL-6 were determined in experimental groups. Value are presented as the mean ± SE. Means with different letters are significantly different at p〈 0.05 by Duncan's multiple range test. Control, group received water without DSS; DSS, group received 3% DSS in drinking water for 5 days; DSS+MT, group received 3% DSS and oral administration with MT water extracts (5 g/kg/day) for 5 days; DSS, dextran sulfate sodium; MT, mulberry twig |
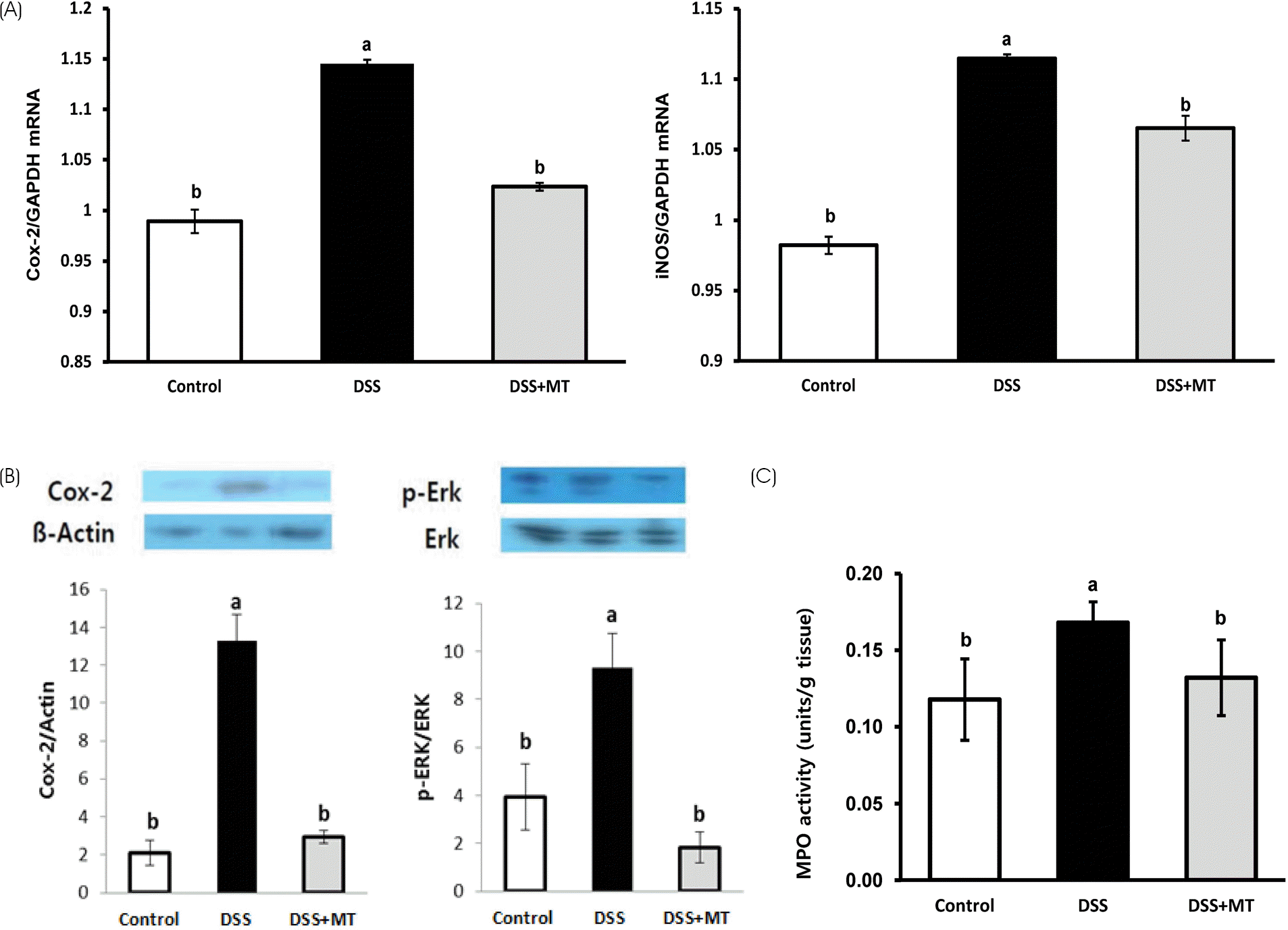 | Fig. 5Colorectal inflammatory gene expression and MPO activity. (A) Cox-2 and iNOS mRNA expression in the distal part of large intestine was analyzed by qRT-PCR. (B) Total protein lysates from the distal part of large intestine were subjected to western analysis with their relevant antibodies. Relative abundance of each band to β-actin or Erk was quantified. (C) MPO activities in colorectal tissues of each group of mice were determined. Value are presented as the mean ± SE. Means with different letters are significantly different at p < 0.05 by Duncan's multiple range test. Control, group received water without DSS; DSS, group received 3% DSS in drinking water for 5 days; DSS+MT, group received 3% DSS and oral administration with MT water extracts (5 g/kg/day) for 5 days; DSS, dextran sulfate sodium; MT, mulberry twig; MPO, myeloperoxidase |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download