Abstract
Purpose:
Methods:
Results:
REFERENCES
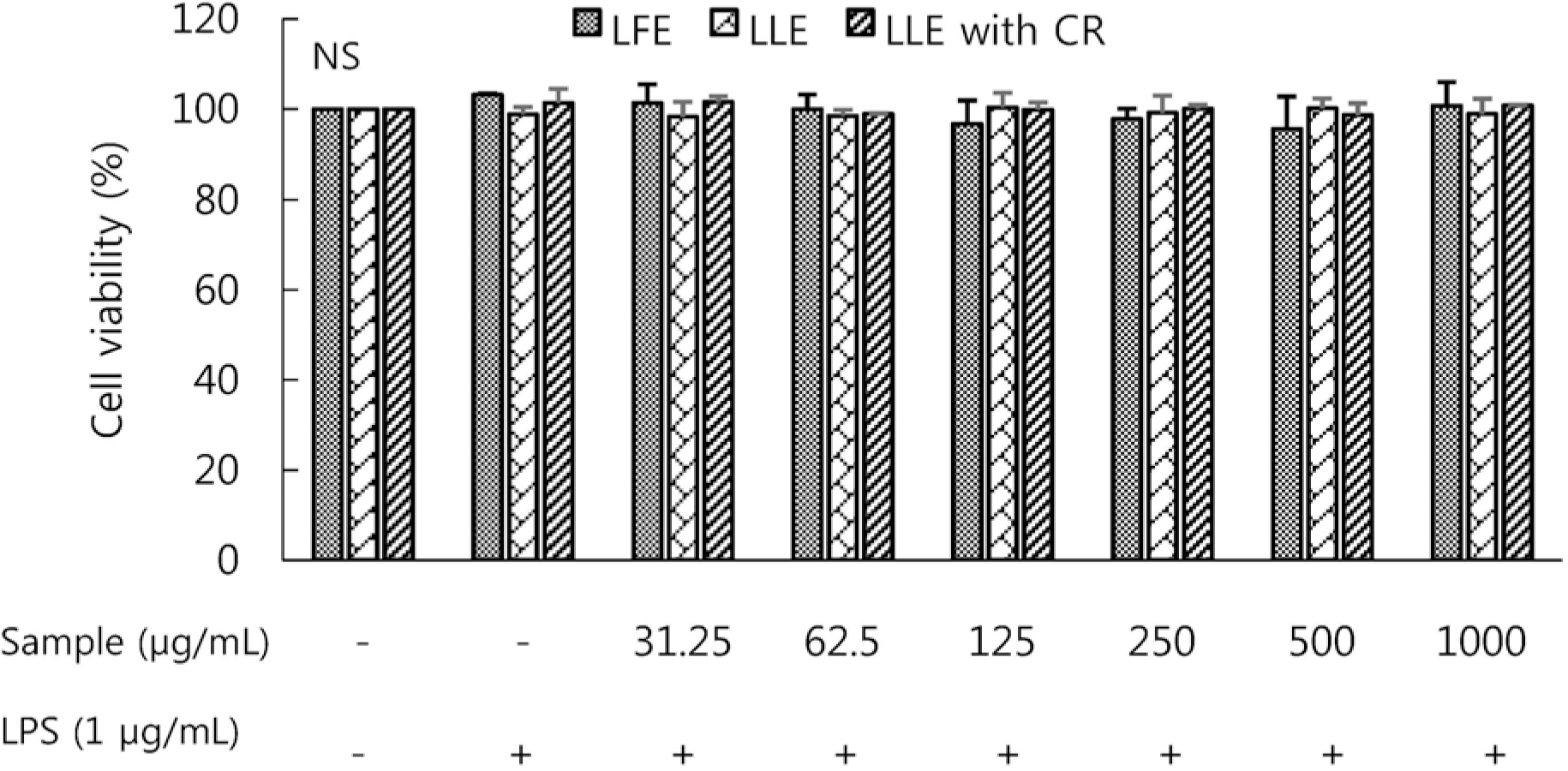 | Fig. 1Effect of Lycium's fruit extracts (LFE) and leaf extracts (LLE), leaf extracts chlorophyll removal (LLE with CR) on cell viability of lipopolysaccharide (LPS)-induced RAW264.7 cells. Cells were treated with LFE, LLE, LLE with CR (31.25, 62.5, 125, 250, 500, 1,000 μg/mL), then with or without LPS (1 μg/mL) for 24 h. The cell proliferation was estimated by the MTT assay with WST system. Each bar represents the mean ± SD. NS: not significantly different by Duncan's multiple range test (p < 0.05). |
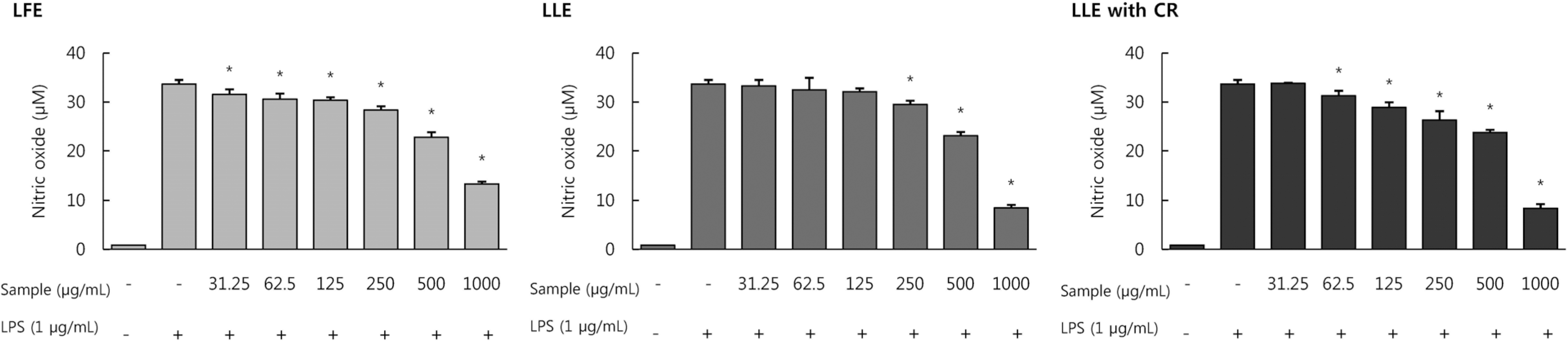 | Fig. 2Effect of Lycium's fruit extracts (LFE) and leaf extracts (LLE), leaf extracts chlorophyll removal (LLE with CR) on the production of nitric oxide (NO) in lipopolysaccharide (LPS)-induced RAW264.7 cells. Cells were treated with LFE, LLE, LLE with CR (31.25, 62.5, 125, 250, 500, 1,000 μg/mL), then with or without LPS (1 μg/mL) for 24 h. The culture supernatant of the treated cells were used to measure NO level. Levels of nitric oxide were determined by Griess reagent. Each bar represents the mean ± SD. Significant values are represented by an asterisk (∗) (p < 0.05 compared to the group treated with LPS alone). |
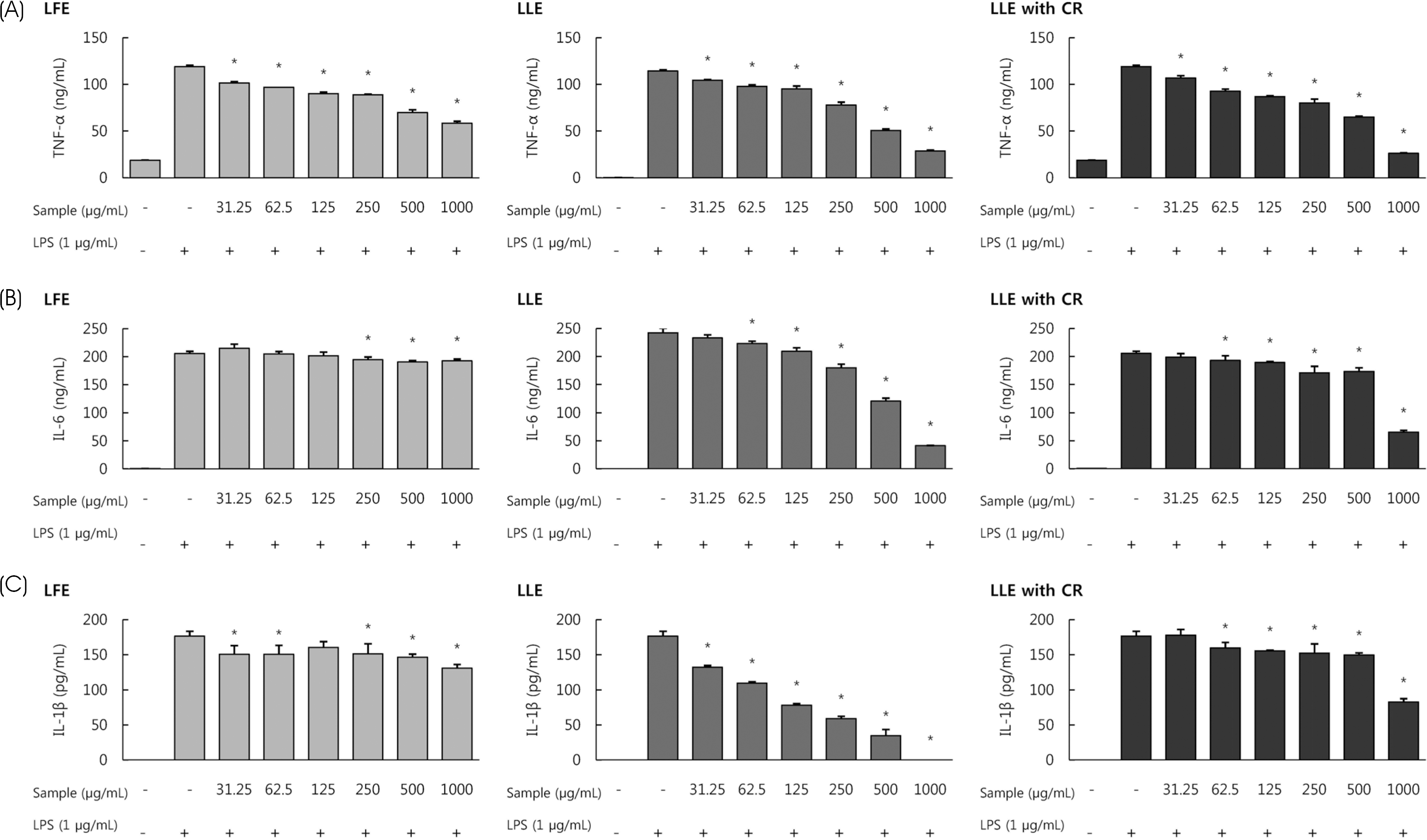 | Fig. 3Effect of Lycium's fruit extracts (LFE) and leaf extracts (LLE), leaf extracts chlorophyll removal (LLE with CR) on the production of TNF-α (A), IL-6 (B), IL-1β (C) in lipopolysaccharide (LPS)-induced RAW264.7 cells. Cells were treated with LFE, LLE, LLE with CR (31.25, 62.5, 125, 250, 500, 1,000 μg/mL), then with or without LPS (1 μg/mL) for 24 h. The levels of pro-inflammatory cytokines in the cell culture supernatant were determined by ELISA. Each bar represents the mean ± SD. Significant values are represented by an asterisk (∗) (p < 0.05 compared to the group treated with LPS alone). |
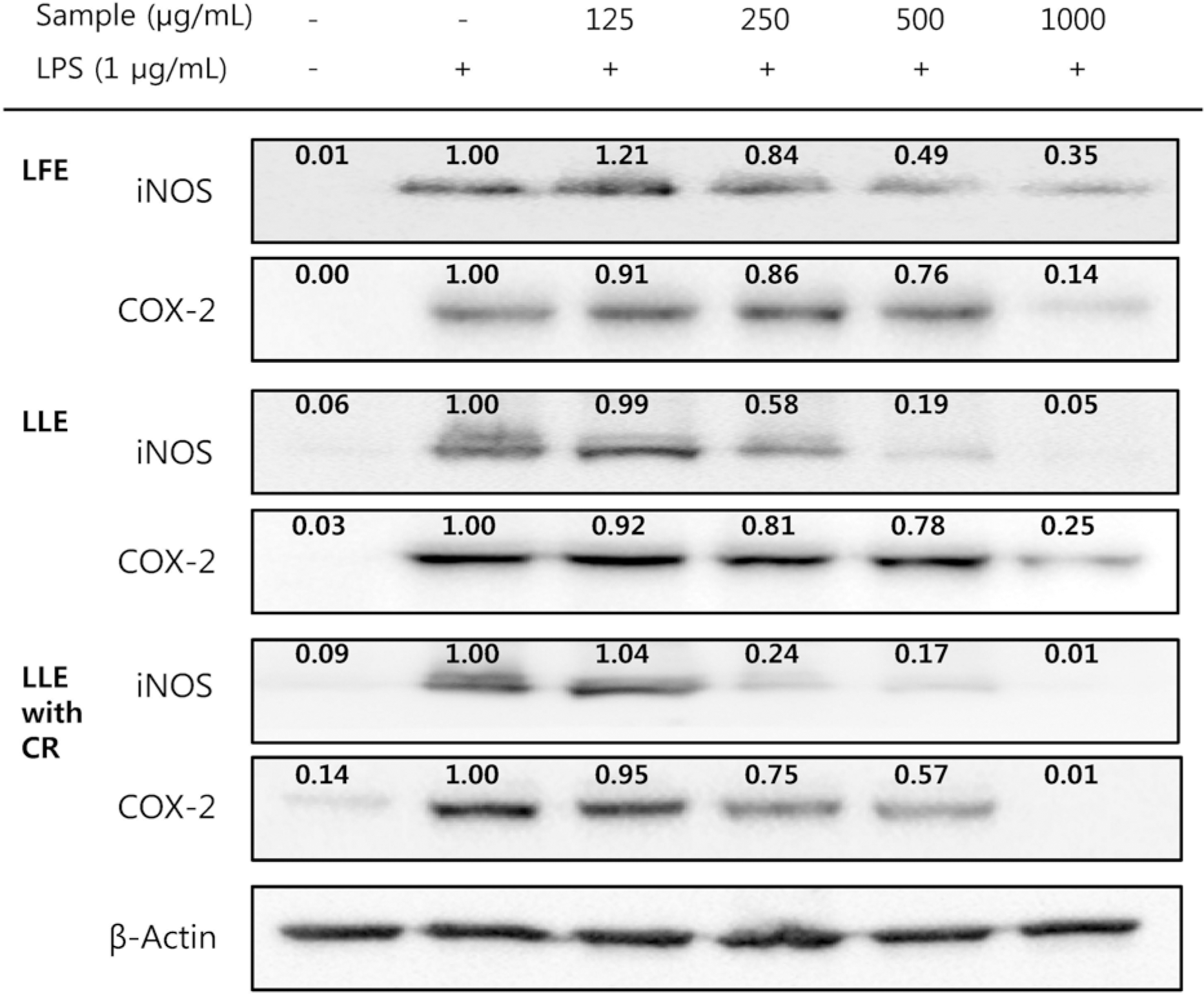 | Fig. 4Effect of Lycium's fruit extracts (LFE) and leaf extracts (LLE), leaf extracts chlorophyll removal (LLE with CR) on iNOS and COX-2 protein expression in lipopolysaccharide (LPS)-induced RAW264.7 cells. Cells were treated with LFE, LLE, LLE with CR (125, 250, 500 and 1,000 μg/mL), then with or without LPS (1 μg/mL) for 24 h. Cell lysates were used for western blot analysis. The levels of protein expression in iNOS and COX-2 were normalized to the β-actin signals. The relative band intensities are indicated above each band. |
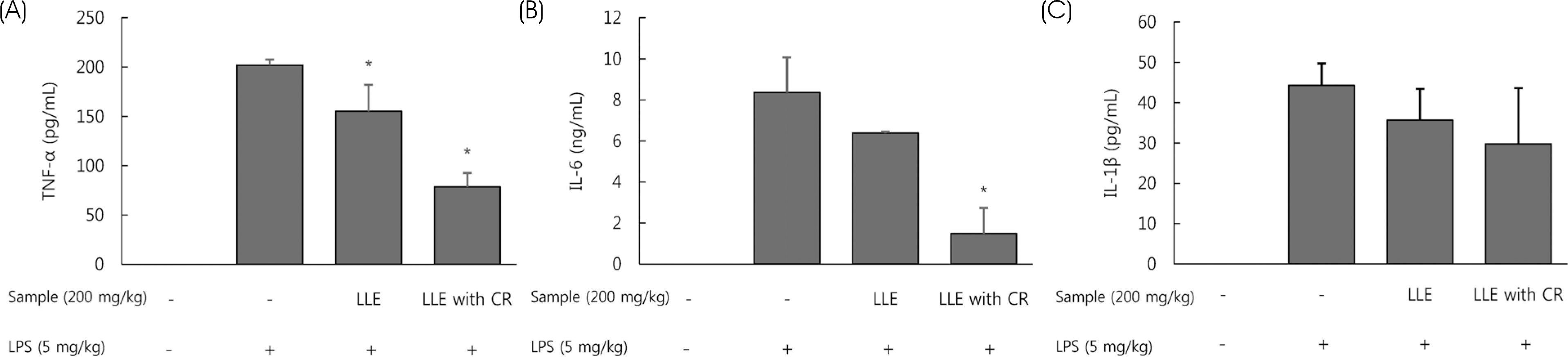 | Fig. 5Effect of Lycium's leaf extracts (LLE), leaf extracts chlorophyll removal (LLE with CR) on the production of serum TNF-α (A), IL-6 (B), IL-1β (C) in LPS-induced BALB/c mice. LLE and LLE with CR (200 mg/kg body weight) was administered orally in mice for seven days, and then challenged intraperitoneally with LPS (5 mg/kg body weight). The levels of pro-inflammatory cytokines in the serum were determined by ELISA. Serum TNF-α, IL-6, IL-1β levels were analyzed 8 h after the last LPS challenge. Each bar represents the mean ± SD. Significant values are represented by an asterisk (∗) (p < 0.05 compared to the group treated with LPS alone). |
Table 1.
1) N (normal), treated with PBS for 8 h; C (control), only treated with LPS 5 mg/kg body weight for 8 h; LLE, treated with Lycium's leaf extracts (200 mg/kg body weight) for seven days and LPS 5 mg/kg body weight for 8 h; LLE with CR, treated with Lycium's leaf extracts chlorophyll removal (200 mg/kg body weight) for seven days and LPS 5 mg/kg body weight for 8 h
Table 2.
| Group1) | Tail DNA (%) | Tail length (μm) | Tail moment |
|---|---|---|---|
| N | 5.40 ± 0.652)3)a | 10.21 ± 1.75a | 0.82 ± 0.17a |
| C | 15.64 ± 5.14b | 46.61 ± 14.17b | 9.48 ± 6.06b |
| LLE | 8.42 ± 1.40a | 20.23 ± 3.91a | 2.29 ± 0.74a |
| LLE with CR | 8.57 ± 1.63a | 14.77 ± 1.29a | 1.93 ± 0.42a |
| F-value | 7.176∗ | 14.438∗ | 4.996∗ |
1) N (normal), treated with PBS for 8 h; C (control), only treated with LPS 5 mg/kg body weight for 8 h; LLE, treated with Lycium's leaf extracts (200 mg/kg body weight) for seven days and LPS 5 mg/kg body weight for 8 h; LLE with CR, treated with Lycium's leaf extracts chlorophyll removal (200 mg/kg body weight) for seven days and LPS 5 mg/kg body weight for 8 h




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download