Abstract
Background
Cognitive therapy may have therapeutic benefit in patients with early Alzheimer's disease (AD).
Case Report
This was a 12-week, single-blind pilot study of 4 patients with AD. The cognitive therapy included exercises for orientation to time and place; memory training, including face-name association, object recall training, and spaced retrieval; visuo-motor organization using software; similarity and ruled based categorization; and behavior modification and sequencing (e.g., making change, paying bills). The regional cerebral metabolic abnormalities and the effects of treatment on cortical metabolic responses were evaluated using 18F-2-fluoro-2-deoxy-D-glucose positron emission tomography (PET). After 12 weeks, the participants showed slight improvement in some neuropsychological measures, and three of them showed increased regional cortical metabolism on brain PET studies.
Alzheimer disease (AD) is a progressive dementing illness affecting approximately 5 to 10% of the population older than 65 years of age and as many as 50% of those older than 85 years of age.1 Initial manifestations of AD include subtle memory loss and forgetfulness, which progress to profound memory loss, cognitive dysfunction, and behavioral and emotional disturbances that may eventually interfere with the patient's ability to perform basic activities of daily living.2 These symptoms result in a patient who requires increasing levels of care throughout the course of the illness and 24-hour supervision in its final stages.2
Treatments for this progressive cognitive impairment are based on cognitive34567891011121314151617181920212223 or pharmacological approaches.24252627 Both types of therapy have been applied separately with different degrees of success. Whereas some pharmacologic therapies have shown early progress in treating the cognitive decline associated with AD,2425 patients and their families often ask if anything can be done at home to help the patient to function better, even if only temporarily.
Numerous cognitive training programs have been developed in an attempt to slow or reverse AD-related cognitive decline and have reported varying degrees of success.34567891011121314151617181920212223 Cognitive activity may delay the clinical onset of AD.18 Most stimulation programs for early AD patients target cognition because some neuronal plasticity and compensation capacity are believed to persist.456910 Although stimulated areas show some benefit, the specificity of the effects, the impact in non-stimulated domains, and long-term maintenance of benefits remain controversial. Most studies with an adequate control group only evaluate the stimulated functions, and there are few long-term reports.202122232425 Cholinesterase inhibitors (ChEI) are of benefit in AD.24252627 A combination of both pharmacologic and cognitive therapy improves cognition in elders with memory complains or dementia.456
The evaluation of treatment effects in AD is a tedious task necessitating large numbers of patients and long observation periods, with inadequate objective tools to measure treatment effects. As impairment of memory and other cognitive disturbances can relate to decreased glucose metabolism in brain regions predominantly affected by AD, improvement in glucose utilization may ameliorate cognitive decline. Regional cerebral metabolic rate of glucose measured by positron emission tomography (PET) may be of value in the preclinical evaluation of drug therapy in this degenerative disorder. Therefore, we tested whether cognitive therapy improved cognitive function, behavioral symptoms, and glucose metabolism using 18F-2-fluoro-2-deoxy-D-glucose (FDG) PET in patients with Alzheimer's disease taking stable maintenance doses of anti-dementia drugs.
Four patients were selected for this study from the Bobath Memorial Hospital cognitive therapy program during 2005 and 2008. Almost all the subjects had over 12 educational years. Specialists from Neurology at Bobath Memorial Hospital had diagnosed these patients as suffering from dementia. They fulfilled the criteria of the Diagnostic and Statistical Manual of Mental Disorders, edition 4, and National Institute of Neurological and Communicative Disorders of Stroke and the Alzheimer's Disease and Related Disorders Association for Alzheimer-type dementia272829 and had brain MRI film checked in the past 1 year. The criteria for exclusion were low educational levels, loss of all speech capacity, requiring assistance for all daily activities, loss of basic psychomotor abilities (e.g., walking, sitting down, etc.), lack of capacity to express emotions adequately, apparent failure of the brain to give orders to the body, and the appearance of generalized and cortical neurological signs and symptoms.
Two different types of treatment were employed in this study:
1) Drug Treatment consisted of symptomatic treatment with donepezil and memantine. The dose of donepezil was between 5 and 10 mg, administered once daily. For the two weeks of medication, 5 mg were administered and thereafter the dose was increased to 10 mg. The medicine was to be taken immediately before going to bed, whether or not a meal was eaten at that time. The dose of memantine was between 10 and 20 mg, administered twice daily. For the two weeks of medication, 10 mg were administered and there after the dose was increased to 20 mg. The medicine was to be taken 30 minutes after having breakfast and dinner. Drug treatment was maintained in stable doses for at least 6 months before cognitive therapy.
2) The cognitive therapy program comprised seven areas of therapy: exercises for orientation to time and place; memory training, including face-name association, object recall training, and spaced retrieval; visuo-motor organization using software; similarity and ruled based categorization; and behavior modification and sequencing (e.g., making change, paying bills). The patients received 45-minute sessions twice per week for 24 sessions. On weekends, families reinforced these exercises at home.
Cognitive functions, such as attention, verbal and visual memory, visuospatial ability, frontal lobe function, and language function were examined using neuropsychological assessment tools. Attention was assessed using forward and backward digit span tests.30 Verbal memory was assessed using Korean version of the Hopkins Verbal Learning Test.31 The Rey Complex Figure Test and Recognition Trial32 were used to assess visuospatial function and visual memory. Neuropsychological assessments primarily associated with the frontal lobe region, including the Controlled Oral Word Association Test33 and the Korean Color-Word Stroop Test,31 were used. Naming ability was assessed using the Korean version of the Boston Naming Test.34 The Lowenstein Occupational Therapy Cognitive Assessment (LOTCA)35 was used to assess cognitive status. The LOTCA consists of 26 sub-tests and can be used to assess six domains (orientation, visual perception, spatial perception, motor praxis, visuomotor organization, thinking operations). The battery takes 30-45 minutes to administer. The entire cognitive function test was performed as a baseline test before the initiation of the cognitive therapy and a follow-up evaluation was performed after the completion of the cognitive therapy. Global measurements, including the Korean Mini-Mental State Examination (K-MMSE), the Clinical Dementia Rating (CDR), sum of boxes of CDR, and the Global Deterioration Scale, were also assessed.36373839 A series of matched pairs t-tests were conducted each group to evaluate significant differences in test scores between the pre- and post-treatment session.
The cortical metabolic response was measured using FDG PET prior to cognitive training and then either after a month in patient 1, or after completion of the total 24 sessions of therapy in the others. Patients fasted for at least 4 hours before the scan, and FDG PET images were acquired 40 min after intravenous administration of 185 mBq of FDG. All images are spatially normalized to the standard template, and regional glucose metabolism was proportionally scaled to the mean uptake in the whole brain. For each patient, the regional hypometabolism on initial or follow-up FDG PET scans were evaluated by comparison with those of age and gender matched healthy controls in a voxel-wise manner using SPM2 (Statistical parametric mapping 2, Wellcome Department of Imaging Neuroscience, London). Changes in cerebral metabolism after cognitive training were tested by subtraction analysis. MRI revealed diffuse brain atrophy but no focal lesions.
After 12 weeks, 2 of 4 patients with milder cognitive impairment showed significant improvement in some neuropsychological measures. The K-MMSE scores improved from 19 to 23, and from 23 to 25, and the total scores of LOTCA improved from 87 to 97, and from 86 to 101, in Patients 1 and 2, during stable donepezil therapy. However, the other 2 patients showed minimal or no change in neuropsychological measures, the K-MMSE scores changed from 15 to 15, and from 16 to 17, and the total scores of LOTCA changed from 58 to 64 and 77 to 78. A slight improvement was observed in some subdomains with detailed neuropsychologic tests (p>0.05) (Table 2, Fig. 1).
Regional hypometabolism through brain regions of the parietal cortex and temporo-occipital cortex was detected at the initial evaluation. Patients 3 and 4, who had higher CDR scores initially, showed widespread hypometabolism in the bilateral frontal cortex as well as parietal and temporal cortex (Fig. 2).
Three patients showed changes in regional metabolism after cognitive therapy. Patient 1, with FDG PET after 4 weeks of cognitive therapy, showed improvements in regional metabolism in the left prefrontal cortex and bilateral anterior temporal cortex on FDG PET. Increased metabolism was predominant in the left temporal and temporo-occipital cortex in patient 2 and the right parietal and temporo-occipital cortex in patient 3, brain regions important in their functional improvement. However, no significant improvement in regional metabolism was detected in patient 4 (Fig. 3).
The current findings indicate that mildly impaired AD patients on a stable dose of anti-dementia medication could benefit from cognitive therapy, demonstrating improvements in measures that assess orientation, learning of face-name associations, speed of processing, and specific functional abilities. A comprehensive stimulation program in AD patients could enhance neuroplasticity processes, reduce cognitive loss, and help patients maintain functional independence through better cognitive performance.4041 In our study, cognitive therapy improved glucose metabolism using brain PET. All of the study participants were diagnosed with AD by brain PET findings as well as clinical findings. Three of them showed improvements in regional metabolism after cognitive therapy, although the regional effects were variable and we could not identify changes in specific brain regions that underlie the tested cognitive functions. Cognitive therapies may improve general brain activity rather than activity in specific brain regions.
Patients showed sustained cognitive function as measured by neuropsychological tests, but did not show improvement. As seen previously, gains from cognitive training are not captured by standardized neuropsychological tests.
The treatment effect was higher in milder patients than moderate stage patients, as evaluated by FDG PET as well as neuropsychologic tests, consistent with previous studies.91011 Although treatment effects were not apparent in moderate to severe AD patients, our study participants cooperated with cognitive therapy and were satisfied with the treatment, suggesting they may still benefit from the therapy.
The influence of education on responsiveness to non-pharmacologic interventions in AD patients is unclear.81142 Education and other activities may increase cognitive reserve and coping with AD. Therefore, given a similar level of clinical severity, AD pathology would be more advanced in the highly educated patients, limiting their learning potential.42 We tried to lessen the influence of educational levels on treatment effects by including participants with similar education levels (mean 14.0±2.3 years).
We did not consider differences of pharmacologic therapy: two patients used donepezil and two patients used memantine, which could have different treatment effects. However, we focused on the effects of cognitive therapy rather than drug treatment.
Limitations of this study include the small sample size and a relatively short follow-up period. The use of neuropsychologic tests may not be valid for AD, and instruments for assessing quality of life would perhaps be more useful. Another potential source of bias was the nonsignificant difference observed in some tests between pre- and post-cognitive training participants. Similarly, any gain in cognitive performance obtained may be lost in a relatively short period.
In conclusion, cognitive therapy may be useful to stabilize or improve cognitive and functional performance of patients with early Alzheimer's disease and increase regional cortical metabolism of the brain. However, due to the small sample sizes in this study, more research is needed into the effectiveness of cognitive interventions for this patient group.
Figures and Tables
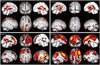 | Fig. 2At baseline, regional hypometabolism through brain regions of the parietal cortex and temporo-occipital cortex was detected. |
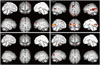 | Fig. 3Three patients showed changes in regional metabolism after cognitive therapy. However, no significant improvement in regional metabolism was detected in patient 4. |
Acknowledgements
This study was supported by a grant of the Korean Health 21 R&D Project, Ministry of Health & Welfare Republic of Korea (A050079).
This study was supported by the IT R&D program of MKE/KEIT [10035434, Assessment Technology of Cognitive Ability in the Elderly].
References
1. Kaplan HI, Sadock BJ. Comprehensive textbook of psychiatry. 6th ed. Baltimore, MD: Williams & Wilkins;1995.

2. Davis RN, Massman PJ, Doody RS. Cognitive intervention in Alzheimer disease: a randomized placebo-controlled study. Alzheimer Dis Assoc Disord. 2001; 15:1–9.

4. Requena C, López Ibor MI, Maestú F, Campo P, López Ibor JJ, Ortiz T. Effects of cholinergic drugs and cognitive training on dementia. Dement Geriatr Cogn Disord. 2004; 18:50–54.

5. Israel L, Melac M, Milinkevitch D, Dubos G. Drug therapy and memory training programs: a double-blind randomized trial of general practice patients with age-associated memory impairment. Int Psychogeriatr. 1994; 6:155–170.

6. Loewenstein DA, Acevedo A, Czaja SJ, Duara R. Cognitive rehabilitation of mildly impaired Alzheimer disease patients on cholinesterase inhibitors. Am J Geriatr Psychiatry. 2004; 12:395–402.

7. Baker R, Bell S, Baker E, Gibson S, Holloway J, Pearce R, et al. A randomized controlled trial of the effects of multi-sensory stimulation (MSS) for people with dementia. Br J Clin Psychol. 2001; 40(Pt 1):81–96.

8. Spector A, Davies S, Woods B, Orrell M. Reality orientation for dementia: a systematic review of the evidence of effectiveness from randomized controlled trials. Gerontologist. 2000; 40:206–212.

9. Clare L, Woods RT, Moniz Cook ED, Orrell M, Spector A. Cognitive rehabilitation and cognitive training for early-stage Alzheimer's disease and vascular dementia. Cochrane Database Syst Rev. 2003; 4:CD003260.

10. Clare L, Woods RT. Cognitive training and cognitive rehabilitation for people with early-stage Alzheimer's disease: a review. Neuropsychol Rehabil. 2004; 5:385–401.

11. Olazarán J, Muñiz R, Reisberg B, Peña-Casanova J, del Ser T, Cruz-Jentoft AJ, et al. Benefits of cognitive-motor intervention in MCI and mild to moderate Alzheimer disease. Neurology. 2004; 63:2348–2353.
12. Spector A, Orrell M, Davies S, Woods RT. Reminiscence therapy for dementia. Cochrane Database Syst Rev. 2000; 4:CD001120.
13. Breuil V, De Rotrou J, Forette F, Tortrat D, Ganansia-Ganem A, Frambourt A, et al. Cognitive stimulation of patients with dementia: preliminary results. Int J Geriatr Psychiatry. 1994; 9:211–217.
14. Teri L, Gibbons LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE, et al. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. JAMA. 2003; 290:2015–2022.
15. Orrell M, Spector A, Thorgrimsen L, Woods B. A pilot study examining the effectiveness of maintenance Cognitive Stimulation Therapy (MCST) for people with dementia. Int J Geriatr Psychiatry. 2005; 20:446–451.

16. Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002; 288:2271–2281.

17. Farina E, Fioravanti R, Chiavari L, Imbornone E, Alberoni M, Pomati S, et al. Comparing two programs of cognitive training in Alzheimer's disease: a pilot study. Acta Neurol Scand. 2002; 105:365–371.
18. Grandmaison E, Simard M. A critical review of memory stimulation programs in Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 2003; 15:130–144.
19. Poon P, Hui E, Dai D, Kwok T, Woo J. Cognitive intervention for community-dwelling older persons with memory problems: telemedicine versus face-to-face treatment. Int J Geriatr Psychiatry. 2005; 20:285–286.
20. Bäckman L. Memory training and memory improvement in Alzheimer's disease: rules and exceptions. Acta Neurol Scand Suppl. 1992; 139:84–89.
21. Bäckman L. Utilizing compensatory task conditions for episodic memory in Alzheimer's disease. Acta Neurol Scand Suppl. 1996; 165:109–113.
22. Camp CJ, Foss JW, O'Hanlon AM, Stevens AB. Memory interventions for persons with dementia. Appl Cogn Psychol. 1996; 10:193–210.
23. Gagnon DL. A review of reality orientation, validation therapy, and reminiscence therapy with the Alzheimer's client. Phys Occup Ther Geriatr. 1996; 14:61–77.
24. Mohs RC, Doody RS, Morris JC, Ieni JR, Rogers SL, Perdomo CA, et al. A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients. Neurology. 2001; 57:481–488.
25. Rogers SL, Doody RS, Mohs RC, Friedhoff LT. Donepezil Study Group. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Arch Intern Med. 1998; 158:1021–1031.
26. Burt T. Donepezil and related cholinesterase inhibitors as mood and behavioral controlling agents. Curr Psychiatry Rep. 2000; 2:473–478.
27. National Institute for Clinical Excellence. Guidance on the use of donepezil, rivastigmine and galantamine for the treatment of Alzheimer's disease (technology appraisal guidance). London: National Institute for Clinical Excellence;2001.

28. American Psychiatric Association. Diagnostic and Statistical. Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association;1994. p. 143–146.

29. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984; 34:939–944.
30. Kang YW, Chin JH, Na DL. A validation study of the Digit Span Test. Korean J Clin Psychol. 2002; 21:911–922.
31. Kang YW, Na DL. Seoul neuropsychological screening battery. Incheon: Human Brain Research & Consulting Co.;2003.
32. Meyers JE, Meyers KR. Rey complex figure test and recognition trial: professional manual. Odessa, FL: Psychological Assessment Resources;1995.
33. Kang YW, Chin JH, Na DL, Lee JH, Park JS. The standardization study of Controlled Oral Word Association Test for the aged. Korean J Clin Psychol. 2000; 19:385–392.
34. Kim HH, Na DL. Korean-Boston naming test (K-BNT). Seoul: Hakjisa;1997.
35. Katz N, Itzkovich M, Averbuch S, Elazar B. Loewenstein Occupational Therapy Cognitive Assessment (LOTCA) battery for brain-injured patients: reliability and validity. Am J Occup Ther. 1989; 43:184–192.

36. Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975; 12:189–198.

37. Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997; 15:300–308.

38. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993; 43:2412–2414.

39. Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982; 139:1136–1139.

40. Becker JT, Mintun MA, Aleva K, Wiseman MB, Nichols T, DeKosky ST. Compensatory reallocation of brain resources supporting verbal episodic memory in Alzheimer's disease. Neurology. 1996; 46:692–700.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download