Abstract
Purpose
Various plants, herbal medicines, and marine foodstuffs have been used in kimchi preparation to improve its overall quality. Teff, which is rich in minerals and starches, facilitates stable blood glucose levels and is well-suited for use in gluten-free products; hence, it can be used to reinforce the mineral composition of kimchi. In this study, we probed the antioxidant activities of hydrolysates prepared by treatment of brown teff with three proteases under different conditions.
Methods
The mineral composition of brown teff was determined by inductively coupled plasma spectrophotometry-mass spectrometry, and we established optimal hydrolysis conditions by determining the total phenol and flavonoid contents of teff hydrolysates obtained using three different proteases (protamax, flavourzyme, and alcalase), two different protease concentrations (1 and 3 wt%), and three different incubation times (1, 2, and 4 h). The antioxidant activity of the hydrolysates was further investigated using 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity, total antioxidant capacity (TAC), and ferrous reducing antioxidant power (FRAP) assays.
Results
Brown teff was rich in I, K, Mg, and Ca, and the highest total phenol content (24.16 µg/mL), total flavonoid content (69.08 µg/mL), and TAC were obtained for 1 wt% protamax treatment. However, the highest DPPH scavenging activity and FRAP values were observed for hydrolysates produced by alcalase and flavourzyme treatments, respectively.
Conclusion
Treatment of brown teff with proteases affords hydrolysates with significantly increased antioxidant activities and high total phenol and flavonoid contents, and these antioxidant activities of teff hydrolysates have the potential to enhance the quality and functionality of kimchi in future applications.
Teff, a fine grain that has been consumed in northeastern Africa since ancient times, provides long-lasting satiation for hunger12 and is predicted to be the next super-grain after quinoa3 and an emerging food trend for 2017.4 Notably, teff is abundant in proteins, vitamin C, dietary fiber, and minerals (especially Ca and Fe), shows high antioxidant activity, and contains resistant starches while being gluten-free; it is widely used in gluten-free products for blood sugar level management.5
In the food industry, various types of enzymatic hydrolysis have been intensively demonstrated and reported. Enzymatic hydrolysis, which is induced by treatment with commercial proteases such as protamax, flavourzyme, and alcalase, was first used to break peptide bonds of proteins and utilized to improve the health benefits of various foods. Numerous studies have shown that hydrolysates obtained by protease treatment exhibit antioxidant, antigenic, and angiotensin-I-converting enzyme (ACE) inhibitory activities. For instance, Sung et al. reported that hydrolysis of buckwheat by seven commercial proteases resulted in antigenicity and allergenicity attenuation,6 while Huang et al. reported that gelatin hydrolysate produced by protease treatment of milkfish showed antioxidant and ACE inhibitory activity.7 The present study is motivated by a lack of available information on the application of commercial proteases to brown teff.
Total phenol and flavonoid contents are known to be positively correlated with antioxidant activity, since these compounds scavenge hydroxyl radicals and thus act as antioxidants; hence, they are often used as indicators of antioxidant activity in many studies.8910 For example, Forsido et al. revealed that the Ethiopian staple grain teff is a valuable food supplement,10 while Inglett et al. investigated the antioxidant activities of free and bound phenolic compounds in ancient grains such as amaranth, quinoa, teff, and buckwheat.11
Recently, kimchi has been regarded worldwide as a functional health food, and the antioxidant, antiobesity, and antidiabetic activities of kimchi have been studied.121314 In addition, kimchi studies with plants, herbal medicines, and marine foodstuffs were conducted to improve the functionality and quality of kimchi. For instance, adding mushrooms, sea tangle extracts, and mustard leaves to kimchi yielded powerful antioxidant properties in the final kimchi.15
In this study, brown teff was selected as a food supplement to increase the quality and health functionality of kimchi. The mineral composition of brown teff was analyzed before enzymatic hydrolysis. Brown teff was hydrolyzed by three commercial proteases (protamax, flavourzyme, and alcalase) at different protease concentrations and incubation times, and color intensity was measured to determine their effects on the final kimchi color. Herein, the total phenol and flavonoid contents of the obtained hydrolysates were determined. In addition, 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity, total antioxidant capacity (TAC), and ferrous reducing antioxidant power (FRAP) assays were performed to investigate the antioxidant activities of the brown teff hydrolysates.
Brown teff of Indonesian origin was purchased from an online market (Handsherb, Yeongcheon, Korea). Protamax, flavourzyme, and alcalase were purchased from Novozyme (Novozyme, Bagsvaerd, Denmark). DPPH, gallic acid, quercetin, and the TAC kit were obtained from Sigma Aldrich (St. Louis, MO, USA). The FRAP kit was purchased from ARBOR ASSAYS (Ann Arbor, MI, USA). Table 1 presents the optimal hydrolysis condition of the commercial proteases.
The brown teff was combusted/pyrolyzed in the presence/absence of air in a furnace at 600℃ for 12 h. The residue was then cooled to room temperature, treated with 6 M HCl, and incubated for 15 h. The obtained mixture was filtered, and a 100 µL filtrate aliquot was diluted with distilled water (DW; 3 mL) and analyzed by inductively coupled plasma spectrophotometry-mass spectrometry (Model #7500a, Agilent Technology, Palo Alto, CA, USA).
To establish optimal hydrolysis conditions, a suspension of brown teff (10 g) in DW (20 mL) was treated with proteases (protamax, flavourzyme, and alcalase) to achieve an enzyme loading of 1 or 3 wt%, and then incubated at 50℃ for 1, 2, or 4 h. Subsequently, proteases were inactivated by 10 min heating at 100℃ and precipitated by addition of 60 vol% aqueous ethanol (20 mL). The obtained mixtures were centrifuged using a Centrifuge 5430R (Eppendorf, Hamburg, Germany) at 3,000 rpm for 30 min, and the supernatant was collected for subsequent experiments. The process of brown teff hydrolysis is illustrated in Fig. 1.
The total phenol contents of brown teff hydrolysates were determined using the Folin-Ciocalteu method16 based on the gallic acid standard curve, and were expressed in mg gallic acid equivalent per gram of extract (mg GAE/g). Absorbance was measured at 750 nm using a SPECTROStar Nano microplate reader (BMG LABTECH, Ortenberg, Germany).
The total flavonoid contents of brown teff hydrolysates were determined using Chang's method17 based on the quercetin standard curve and were expressed in mg quercetin equivalent per gram of extract (mg EQ/g). Absorbance was measured at 425 nm using a microplate reader.
The antioxidant activities of brown teff hydrolysates were analyzed using a modified DPPH radical scavenging assay.18 In particular, absorbance was measured at 515 nm using a microplate reader, and the inhibition percentage (PI) was calculated as PI = [(Absorbance of DW − absorbance of the brown teff sample)/absorbances of DW] × 100%.
TAC values of brown teff hydrolysates were measured based on the Trolox standard curve using a colorimetric assay kit. Absorbance was measured at 570 nm using a microplate reader. TAC values were expressed as nM.
Table 2 shows the mineral contents of brown teff, demonstrating that this grain is rich in iodine (I; 365.4 mg/100 g), potassium (K; 353.5 mg/100 g), calcium (Ca), and magnesium (Mg). These results were consistent with other studies showing the high calcium content of teff. However, sodium (Na), iron (Fe), and zinc (Zn) contents were lower than 10 mg/100 g. Unexpectedly, this result was inconsistent with other studies that have reported high iron contents in brown teff.
Fig. 1 shows brown teff hydrolysates prepared under different conditions, revealing that color intensity decreased in the order of protamax > flavourzyme > alcalase and increased with increasing enzyme loading. In addition, the color of brown teff was softened by hydrolysis, leading to an easier application to kimchi. On average, 7 mL of brown teff hydrolysate was obtained from 10 g of brown teff, 20 mL of DW, and 20 mL of 60 vol% ethanol.
Fig. 2A shows that the highest total phenol content was observed for the hydrolysate prepared using 1 wt% protamax and an incubation time of 4 h (24.16 µg/mL; p < 0.01). At an enzyme loading of 1 wt% and constant incubation time, the total phenol content decreased in the order of protamax > flavourzyme > alcalase. However, no significant differences between enzymes were observed at loadings of 3 wt% (Fig. 2B). In all cases, the total phenol content exceeded 19 µg/mL. Thus, the above results indicate that protamax-catalyzed hydrolysis was the most efficient in this particular study.
The total flavonoid content of teff hydrolysates showed a variation trend similar to that of total phenol content and was 30.35 ~ 69.18 µg/mL, with the highest value in the case of 4 h 1 wt% protamax treatment (Fig. 3A, p < 0.01). Notably, incubation with 3 wt% alcalase for 1 h and 2 h significantly increased the total flavonoid content of brown teff hydrolysate, as shown in Fig. 3B (p < 0.05, p < 0.01, respectively). Thus, as in the case of total phenol content, protamax was concluded to be the most efficient enzyme for teff hydrolysis, and brown teff hydrolysates were concluded to exhibit significant antioxidant activity.
The antioxidant activities of teff hydrolysates were further studied based on DPPH scavenging activity, TAC, and FRAP assays. Both 1 and 3 wt% alcalase treatments significantly increased the DPPH scavenging activity of brown teff hydrolysate compared to protamax and flavourzyme treatment (p < 0.01, p < 0.0001). At an enzyme loading of 1 wt%, the DPPH scavenging activity of hydrolysates decreased in the order of alcalase > flavourzyme > protamax (Fig. 4A), while the highest DPPH scavenging activity (74.08%, p < 0.0001) was observed for 1 h incubation with 3 wt% alcalase (Fig. 4B).
As shown in Fig. 5A, the highest TAC values were observed for the hydrolysate prepared by 1 h incubation with 1 wt% protamax (6,447.14 nM; p < 0.01). Incubation with 3 wt% protamax for 2 h significantly increased the TAC values of brown teff hydrolysate (Fig. 5B, p < 0.0001). All TAC values exceeded 4,000 nM.
Fig. 6 shows the FRAP values of brown teff hydrolysates, revealing that both 1 and 3 wt% flavourzyme treatments resulted in elevated FRAP values (p < 0.01 and p < 0.05, respectively). Notably, high FRAP values were observed in all protease treatment groups, with a maximum value of 1,809.36 µM observed for 4 h incubation with 3 wt% alcalase. The above FRAP value was similar to that observed for 2 h incubation with 1 wt% flavourzyme (1,805.43 µM). Taken together, the results of the above assays indicate that the antioxidant activity of brown teff hydrolysate depends on the type of protease and quantitation method.
The recent popularization of teff as a functional grain has resulted in numerous investigations and inspired the development of various recipes for its preparation. In particular, the present study was performed to characterize the functional properties of brown teff, especially its antioxidant activity.
As commented on previously, protamax, flavourzyme, and alcalase are widely used in the food industry to improve the functional, nutritional and flavoring properties of proteins. Protamax and flavourzyme are mixtures of endoand exopeptidases, and derived from Bacillus sp. and Aspergillus oryzae, respectively. Endopeptidases break peptide bonds of nonterminal amino acids, while exopeptidases break those of terminal amino acids such as amino and carboxyl residues. As an endopeptidase, alcalase is specifically produced by Bacillus licheniformis.19 These proteases react under neutral or slightly acidic conditions20 and the properties and functionalities of hydrolysates may vary depending on the characteristics of their proteases. Therefore, the choice of the most efficient protease for a specific food application is important.
Mineral composition analysis revealed that teff was particularly rich in P, K, Mg, and Ca, which is in agreement with the results of Forsido et al., who reported that teff, wheat, corn, and tapioca are rich in P, Mg, Mn, and Cu.10 Subsequently, brown teff was hydrolyzed with different proteases (protamax, flavourzyme, and alcalase) under different conditions, and the obtained hydrolysates were characterized in terms of antioxidant activity. The color and yield of these hydrolysates were slightly dependent on protease identity. Although the chosen proteases have been intensively used in the field of food chemistry, protease treatment has not yet been applied to teff.2122232425 For instance, Uraipong et al. hydrolyzed rice bran using four proteases (protamax, flavourzyme, alcalase, and neutrase) to investigate its beneficial health effects, revealing that the fractionated hydrolysate featured α-amylase and α-glucosidase inhibitory activities.21 In another study, Noh et al. investigated the products of red crab (Chionoecetes japonicus) shell powder hydrolysis by commercial proteases (protamax, flavourzyme, alcalase, neutrase, protease A, and protease M) to determine their soluble protein contents and amino acid concentrations.22 Taken together, the proper utilization of proteases is known to enhance the functional activity of food.
Total phenol content represents the content of various phenolic compounds such as gallic acid, chlorogenic acid, epicatechin, rutin, naringin, and quercetin, which are all known to exhibit powerful antioxidant activity.26 Hence, the total phenol content of brown teff hydrolysates was measured in this study. On average, the total phenol content of brown teff hydrolysate slightly exceeded 19 µg/mL, with the highest value of 24.16 µg/mL observed in 1 wt% protamax incubation for 4 h. In another study, the total phenol content, phenolic profile, and antioxidant activity were shown to be affected by the heating method (i.e., water cooker, rice cooker, or sous-vide),27 implying that appropriate treatment of white or brown teff can result in an increased total phenol content and/or antioxidant activity.
Total flavonoid (flavone, flavonol, and tannin) content is also involved in antioxidant activity, since flavonoids are known to act as antioxidants both in vitro and in vivo.2829 In the present study, the maximum total flavonoid content (69.08 µg/mL) was observed in 1 wt% protamax incubation for 4 h. Based on the total phenol and flavonoid contents of brown teff hydrolysates, they were expected to exhibit potent antioxidant activity. However, total phenol and flavonoid content of brown teff hydrolysate showed different results at different time points according to the protease used. These results might be due to the different compositions of phenolic components and flavonoid components depending on the protease type, concentration, and incubation time. In future studies, phenolic and flavonoid components should be studied simultaneously.
The antioxidant activities of brown teff hydrolysates were determined by DPPH, TAC, and FRAP assays, which afforded different results. In particular, the lowest DPPH radical scavenging activity3031 was observed in the case of 2 h incubation with 1 wt% protamax, while the highest activity was observed for 1 h incubation with 3 wt% alcalase. Similarly to the DPPH assay, the TAC assay also determines free radical scavenging activity. However, the results of the TAC assay were different from those of the DPPH assay. Protamax-catalyzed hydrolysis significantly increased the TAC value of brown teff hydrolysate, whereas much lower activities were observed when flavourzyme and alcalase were used. Thus, the results of the TAC assay implied that the antioxidant activity of brown teff can be increased by protamax-catalyzed hydrolysis and were in agreement with the results of total phenol and flavonoid content analyses. The correlation between total phenol and TAC values were only positive in the case of 1 wt% protamax treatment, whereas positive correlation was present for 3 wt% protamax treatment. Although the correlation was not large, antioxidant activity of brown teff hydrolysate was strongly revealed in this study.
Lastly, FRAP assay is a method of measuring the antioxidant activity of a sample based on its reducing power. High FRAP values were observed in all brown teff hydrolysates, with the maximum being in the case of flavourzyme treatment. Antioxidant activity of brown teff hydrolysate was found in all treatment groups, but differed depending on protease type and assay method. Taken together, the addition of brown teff hydrolysate is expected to improve the quality and functionality of kimchi.
Brown teff was hydrolyzed using different proteases (protamax, flavourzyme, and alcalase), protease loadings (1 and 3 wt%), and incubation times (1, 2, and 4 h), with the highest total phenol and flavonoid contents observed in the case of 1 wt% protamax treatment. Notably, the antioxidant activities of brown teff hydrolysate were slightly different according to the antioxidant assay method. Taken together, brown teff has high potential as a food supplement with potent antioxidant activity. These results suggest the addition of teff hydrolysate to kimchi could enhance the health functionality and quality of kimchi, leading to increased consumer acceptability of kimchi.
Figures and Tables
 | Fig. 2Total phenol contents of brown teff hydrolysates produced by (A) 1 wt% and (B) 3 wt% treatment with protamax (P), flavourzyme (F), and alcalase (A). NS: not significantly different; * P < 0.05, ** P < 0.01. |
 | Fig. 3Total flavonoid contents of brown teff hydrolysates produced by (A) 1 wt% and (B) 3 wt% treatment with protamax (P), flavourzyme (F), and alcalase (A). NS: not significantly different; * P < 0.05, ** P < 0.01, *** P < 0.001. |
 | Fig. 4DPPH radical scavenging activities of brown teff hydrolysates produced by (A) 1 wt% and (B) 3 wt% treatment with protamax (P), flavourzyme (F), and alcalase (A). NS: not significantly different; * P < 0.05, ** P < 0.01, *** P < 0.001. |
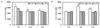 | Fig. 5Total antioxidant capacity values of brown teff hydrolysates produced by (A) 1 wt% and (B) 3 wt% treatment with protamax (P), flavourzyme (F), and alcalase (A). NS: not significantly different; * P < 0.05, ** P < 0.01, *** P < 0.001. |
References
1. Shumoy H, Raes K. Tef: the rising ancient cereal: what do we know about its nutritional and health benefits. Plant Foods Hum Nutr. 2017; 72(4):335–344.

2. Collar C, Jiménez T, Conte P, Fadda C. Impact of ancient cereals, pseudocereals and legumes on starch hydrolysis and antiradical activity of technologically viable blended breads. Carbohydr Polym. 2014; 113:149–158.

3. Provost C, Jobson E. Move over quinoa, Ethiopia's teff poised to be next big super grain. The Guardian. 2014. 01. 23.
4. O'Connor A. Is teff the new super grain? The New York Times. 2016. 08. 16.
5. Rybicka I, Krawczyk M, Stanisz E, Gliszczyńska-Świgło A. Selenium in gluten-free products. Plant Foods Hum Nutr. 2015; 70(2):128–134.

6. Sung DE, Lee J, Han Y, Shon DH, Ahn K, Oh S, Do JR. Effects of enzymatic hydrolysis of buckwheat protein on antigenicity and allergenicity. Nutr Res Pract. 2014; 8(3):278–283.

7. Huang CY, Tsai YH, Hong YH, Hsieh SL, Huang RH. Characterization and antioxidant and angiotensin I-converting enzyme (ACE)-inhibitory activities of gelatin hydrolysates prepared from extrusion-pretreated milkfish (Chanos chanos) scale. Mar Drugs. 2018; 16(10):346.

8. Subedi L, Timalsena S, Duwadi P, Thapa R, Paudel A, Parajuli K. Antioxidant activity and phenol and flavonoid contents of eight medicinal plants from Western Nepal. J Tradit Chin Med. 2014; 34(5):584–590.

9. Jing L, Ma H, Fan P, Gao R, Jia Z. Antioxidant potential, total phenolic and total flavonoid contents of Rhododendron anthopogonoides and its protective effect on hypoxia-induced injury in PC12 cells. BMC Complement Altern Med. 2015; 15:287.

10. Forsido SF, Rupasinghe HP, Astatkie T. Antioxidant capacity, total phenolics and nutritional content in selected ethiopian staple food ingredients. Int J Food Sci Nutr. 2013; 64(8):915–920.

11. Inglett GE, Chen D, Liu SX. Antioxidant activities of selective gluten free ancient grains. Food Nutr Sci. 2015; 6(7):612–621.

12. Kim BK, Choi JM, Kang SA, Park KY, Cho EJ. Antioxidative effects of Kimchi under different fermentation stage on radical-induced oxidative stress. Nutr Res Pract. 2014; 8(6):638–643.

13. Lee KH, Song JL, Park ES, Ju J, Kim HY, Park KY. Anti-obesity effects of starter fermented kimchi on 3T3-L1 adipocytes. Prev Nutr Food Sci. 2015; 20(4):298–302.

14. Lee HA, Song YO, Jang MS, Han JS. Alleviating effects of baechu kimchi added Ecklonia cava on postprandial hyperglycemia in diabetic mice. Prev Nutr Food Sci. 2013; 18(3):163–168.

15. Ha SH, Kang SA. Effect of addition of mushroom and sea tangle extracts and mustard leaf on anti-oxidant properties of Kimchi. Korean J Food Nutr. 2018; 31(4):471–477.
16. Durazzo A, Turfani V, Azzini E, Maiani G, Carcea M. Phenols, lignans and antioxidant properties of legume and sweet chestnut flours. Food Chem. 2013; 140(4):666–671.

17. Chang CC, Yang MH, Wen HM, Cherrn JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002; 10(3):178–182.

18. Villaño D, Fernández-Pachón MS, Moyá ML, Troncoso AM, García-Parrilla MC. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta. 2007; 71(1):230–235.

19. Kristinsson HG, Rasco BA. Fish protein hydrolysates: production, biochemical, and functional properties. Crit Rev Food Sci Nutr. 2000; 40(1):43–81.

20. Šližytė R, Mozuraitytė R, Martinez-Alvarez O, Falch E, Fouchereau-Peron M, Rustad T. Functional, bioactive and antioxidative properties of hydrolysates obtained from cod (Gadus morhua) backbones. Process Biochem. 2009; 44(6):668–677.

21. Uraipong C, Zhao J. Rice bran protein hydrolysates exhibit strong in vitro α-amylase, β-glucosidase and ACE-inhibition activities. J Sci Food Agric. 2016; 96(4):1101–1110.
22. Noh KH, Min KH, Seo BY, Kim SH, Seo YW, Song YS. Characteristics of protein from red crab (Chionoecetes japonicus) shell by commercial proteases. Korean J Nutr. 2012; 45(5):429–436.

23. Gao Q, Smith JC, Tsopmo A. Optimized protamex digested oat bran proteins: antioxidant properties and identification of new peptides. Austin J Nutr Food Sci. 2014; 2(10):1053.
24. Nguyen HQ, Dong DA. Release bioactive peptides from enzymatic hydrolysed soybean by alcalase and protamex using response surface methodology. J Sci Technol. 2017; 55(2):137–149.
25. Nguyen HT, Sylla KS, Randriamahatody Z, Donnay-Moreno C, Moreau J, Tran LT, Bergé JP. Enzymatic hydrolysis of yellowfin tuna (Thunnus albacares) by-products using protamex protease. Food Technol Biotechnol. 2011; 49(1):48–55.
26. Soobarttee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T. Phenolics as potential antioxidant therapeutic agents: mechanism and action. Mutt Res. 2005; 579(1-2):203–213.
27. Koubová E, Mrázková M, Sumczynski D, Orsavová J. In vitro digestibility, free and bound phenolic profiles and antioxidant activity of thermally treated Eragrostis tef L. J Sci Food Agric. 2018; 98(8):3014–3021.

28. Geetha S, Sai Ram M, Mongia SS, Singh V, Ilavazhagan G, Sawhney RC. Evaluation of antioxidant activity of leaf extract of Seabuckthorn (Hippophae rhamnoides L.) on chromium(VI) induced oxidative stress in albino rats. J Ethnopharmacol. 2003; 87(2-3):247–251.

29. Shimoi K, Masuda S, Shen B, Furugori M, Kinae N. Radioprotective effects of antioxidative plant flavonoids in mice. Mutat Res. 1996; 350(1):153–161.

30. Prakash A. Antioxidant activity. Med Lab Anal Prog. 2001; 19(2):1–6.
31. Sendra JM, Sentandreu E, Navarro JL. Reduction kinetics of the free stable radical 2, 2-diphenyl-1-picrylhydrazyl (DPPH•) for determination of the antiradical activity of citrus juices. Eur Food Res Technol. 2006; 223(5):615–624.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download