Abstract
Purpose
Sargassum horneri (S. horneri) is a species of brown macroalgae that is common along the coast of Japan and Korea. The present study investigated the immuno-modulatory effects of different types of S. horneri extracts in RAW264.7 macrophages. Methods: S. horneri was extracted by three different methods, hot water extraction, 50% ethanol extraction, and supercritical fluid extraction. Cell viability was then measured by MTT assay, while the production levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and nitric oxide (NO) were measured by enzyme-linked immunosorbent assay and Griess assay, respectively. The expression and activation levels of inducible NO synthase (iNOS), mitogen-activated protein kinase (MAPK) and nuclear factor κB (NF-κB) were examined by western blot analysis. Results: The three different S. horneri extracts were nontoxic against RAW 264.7 cells up to 50 μg/mL, among which treatment with hot water extract (HWE) of S. horneri significantly enhanced the production of TNF-α, IL-6, and NO in a dose-dependent manner. Hot water extract of S. horneri also increased the expression level of iNOS, suggesting that up-regulation of iNOS expression by HWE of S. horneri was responsible for the induction of NO production. In addition, treatment of RAW 264.7 macrophages with HWE of S. horneri increased the phosphorylation levels of ERK, p38 and JNK. Furthermore, the activation and subsequent nuclear translocation of NF-κB was enhanced upon treatment with HWE of S. horneri, indicating that HWE of S. horneri activates macrophages to secrete TNF-α, IL-6 and NO and induces iNOS expression via activation of the NF-κB and MAPKs signaling pathways. Conclusion: Taken together, these findings suggest that HWE of S. horneri possesses potential as a functional food with immunomodulatory activity.
Go to : 
References
1. Cha JH, Lim EM. Effects of Gardeniae fructus on cytokines in mouse macrophage. J Korean Obstet Gynecol. 2014; 27(1):1–16.

2. Erwig LP, Rees AJ. Macrophage activation and programming and its role for macrophage function in glomerular inflammation. Kidney Blood Press Res. 1999; 22(1–2):21–25.
3. Jiang MH, Zhu L, Jiang JG. Immunoregulatory actions of polysaccharides from Chinese herbal medicine. Expert Opin Ther Targets. 2010; 14(12):1367–1402.

4. Yuan H, Song J, Li X, Li N, Dai J. Immunomodulation and antitumor activity of kappa-carrageenan oligosaccharides. Cancer Lett. 2006; 243(2):228–234.
5. Hong HD, Cho CW, Rhee YK, Choi HD, Lee HS. Status of technology development using immune-modulating polysaccharide. Food Sci Ind. 2012; 45(1):2–11.
6. Yan C, Yin Y, Zhang D, Yang W, Yu R. Structural characterization and in vitro antitumor activity of a novel polysaccharide from Taxus yunnanensis. Carbohydr Polym. 2013; 96(2):389–395.

7. Brown ES, Allsopp PJ, Magee PJ, Gill CI, Nitecki S, Strain CR, McSorley EM. Seaweed and human health. Nutr Rev. 2014; 72(3):205–216.

8. Kim NG. Effects of temperature, photon irradiance, and photoperiod on the growth of embryos of Sargassum horneri in laboratory culture. Korean J Fish Aquat Sci. 2015; 48(1):76–81.

9. Liu L, Heinrich M, Myers S, Dworjanyn SA. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: a phytochemical and pharmacological review. J Ethnopharmacol. 2012; 142(3):591–619.

10. Preeprame S, Hayashi K, Lee JB, Sankawa U, Hayashi T. A novel antivirally active fucan sulfate derived from an edible brown alga, Sargassum horneri. Chem Pharm Bull (Tokyo). 2001; 49(4):484–485.

12. Food and Agriculture Organization of the United Nations (IT). A guide to the seaweed industry: FAO fisheries technical paper 441. 8. Seaweeds used as human food [Internet]. Rome: Food and Agriculture Organization of the United Nations;2003. [cited 2018 Dec. 16]. Available from:. http://www.fao.org/docrep/006/y4765e/y4765e0b.htm.
13. Cardoso SM, Pereira OR, Seca AM, Pinto DC, Silva AM. Seaweeds as preventive agents for cardiovascular diseases: from nutrients to functional foods. Mar Drugs. 2015; 13(11):6838–6865.

14. Yamaguchi M. Regulatory mechanism of food factors in bone metabolism and prevention of osteoporosis. Yakugaku Zasshi. 2006; 126(11):1117–1137.

15. Shao P, Chen X, Sun P. Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri. Carbohydr Polym. 2014; 105:260–269.

16. Shao P, Liu J, Chen X, Fang Z, Sun P. Structural features and antitumor activity of a purified polysaccharide extracted from Sargassum horneri. Int J Biol Macromol. 2015; 73:124–130.

17. Yoshimura A. Signal transduction of inflammatory cytokines and tumor development. Cancer Sci. 2006; 97(6):439–447.

18. Schorey JS, Cooper AM. Macrophage signalling upon mycobacterial infection: the MAP kinases lead the way. Cell Microbiol. 2003; 5(3):133–142.

19. Gaestel M, Mengel A, Bothe U, Asadullah K. Protein kinases as small molecule inhibitor targets in inflammation. Curr Med Chem. 2007; 14(21):2214–2234.

20. Yoon TJ. Effect of water extracts from root of Taraxacum officinale on innate and adaptive immune responses in mice. Korean J Food Nutr. 2008; 21(3):275–282.
21. Izadpanah K, Freyer D, Weber JR, Braun JS. Brain parenchymal TNF-α and IL-1β induction in experimental pneumococcal meningitis. J Neuroimmunol. 2014; 276(1–2):104–111.

22. Byun EH. Comparison study of immunomodulatory activity of polysaccharide and ethanol extracted from Sargassum fulvellum. J Korean Soc Food Sci Nutr. 2015; 44(11):1621–1628.

23. Cha JH, Kim YS, Lee EM. Effects of Prunellae spica water extract on immune response in macrophage cells. J Orient Obstet Gynecol. 2010; 23(3):91–100.
24. Chiou WF, Chou CJ, Chen CF. Camptothecin suppresses nitric oxide biosynthesis in RAW 264.7 macrophages. Life Sci. 2001; 69(6):625–635.

25. Hibbs JB Jr, Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988; 157(1):87–94.

26. Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000; 406(6797):782–787.

27. Rosadini CV, Kagan JC. Early innate immune responses to bacterial LPS. Curr Opin Immunol. 2017; 44:14–19.

28. Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003; 24(7):358–363.

29. Peroval MY, Boyd AC, Young JR, Smith AL. A critical role for MAPK signalling pathways in the transcriptional regulation of toll like receptors. PLoS One. 2013; 8(2):): e51243.

30. Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997; 388(6640):394–397.

31. Kim ME, Jung YC, Jung I, Lee HW, Youn HY, Lee JS. Anti-inflammatory effects of ethanolic extract from Sargassum horneri (Turner) C. Agardh on lipopolysaccharide-stimulated macrophage activation via NF-κB pathway regulation. Immunol Invest. 2015; 44(2):137–146.
Go to : 
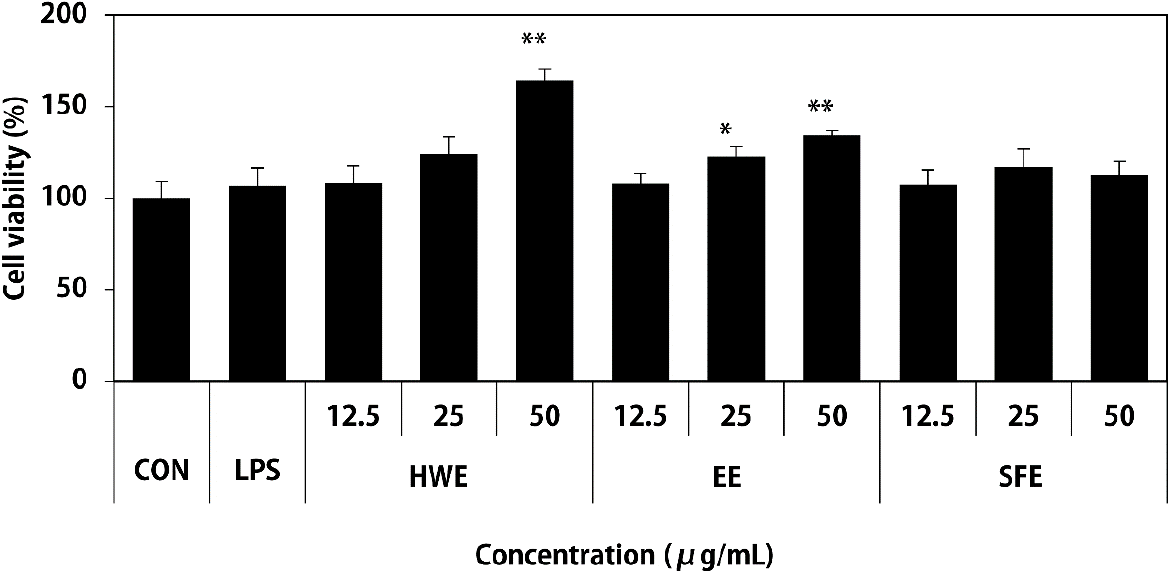 | Fig. 1.Effects of different types of Sargassum horneri extracts on the cell viability of Raw264.7 macrophages. HWE, EE, and SFE were treated at the concentration of 12.5, 25, and 50 μg/mL and LPS (1 μg/mL) used as a positive control. After 24 h, cell proliferation was determined by MTT assay. Results are expressed as the mean ± SD (n = 3). Statistical analysis was performed using Student's two tails t-test with a significant level of ∗ p < 0.05, and ∗∗ p < 0.01 compared to control (CON) group. HWE, hot water extract; EE, ethanol extract; SFE, supercritical fluid extract |
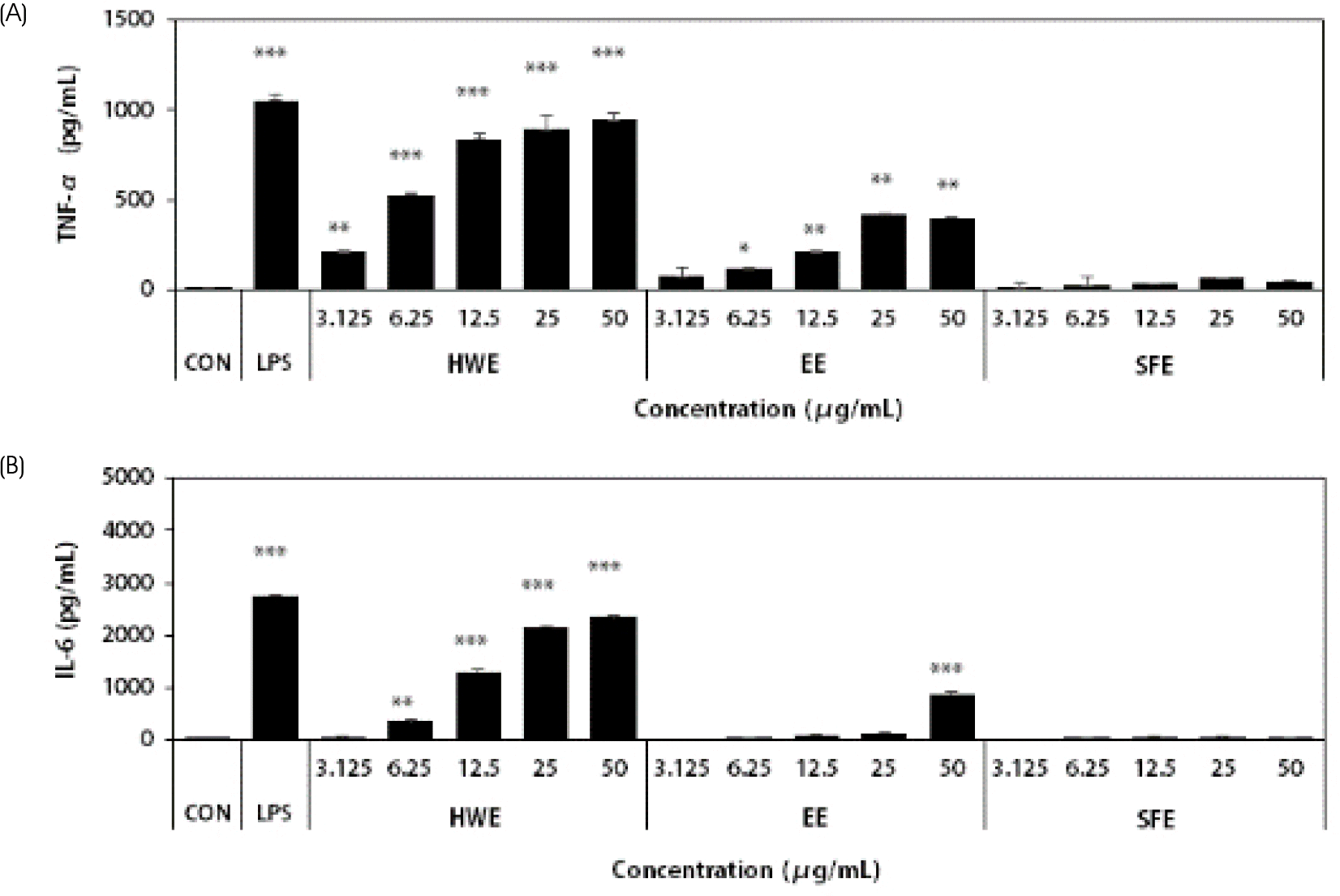 | Fig. 2.Effects of different types of Sargassum horneri extracts on the production of TNF-α (A) and IL-6 (B) in Raw264.7 macrophages. HWE, EE, and SFE were treated at the concentration of 3.125, 6.25, 12.5, 25, and 50 μg/mL in RAW 264.7 cells. LPS (1 μg/mL) used as a positive control. After 24hr, cytokine productions in culture supernatant were investigated by using ELISA kits. Results are expressed as the mean ± SD (n = 3). Statistical analysis was performed using Student's two tails t-test with a significant level of ∗ p < 0.05, ∗∗ p < 0.01, and ∗∗∗ p < 0.001, compared to control (CON) group. HWE, hot water extract; EE, ethanol extract; SFE, supercritical fluid extract |
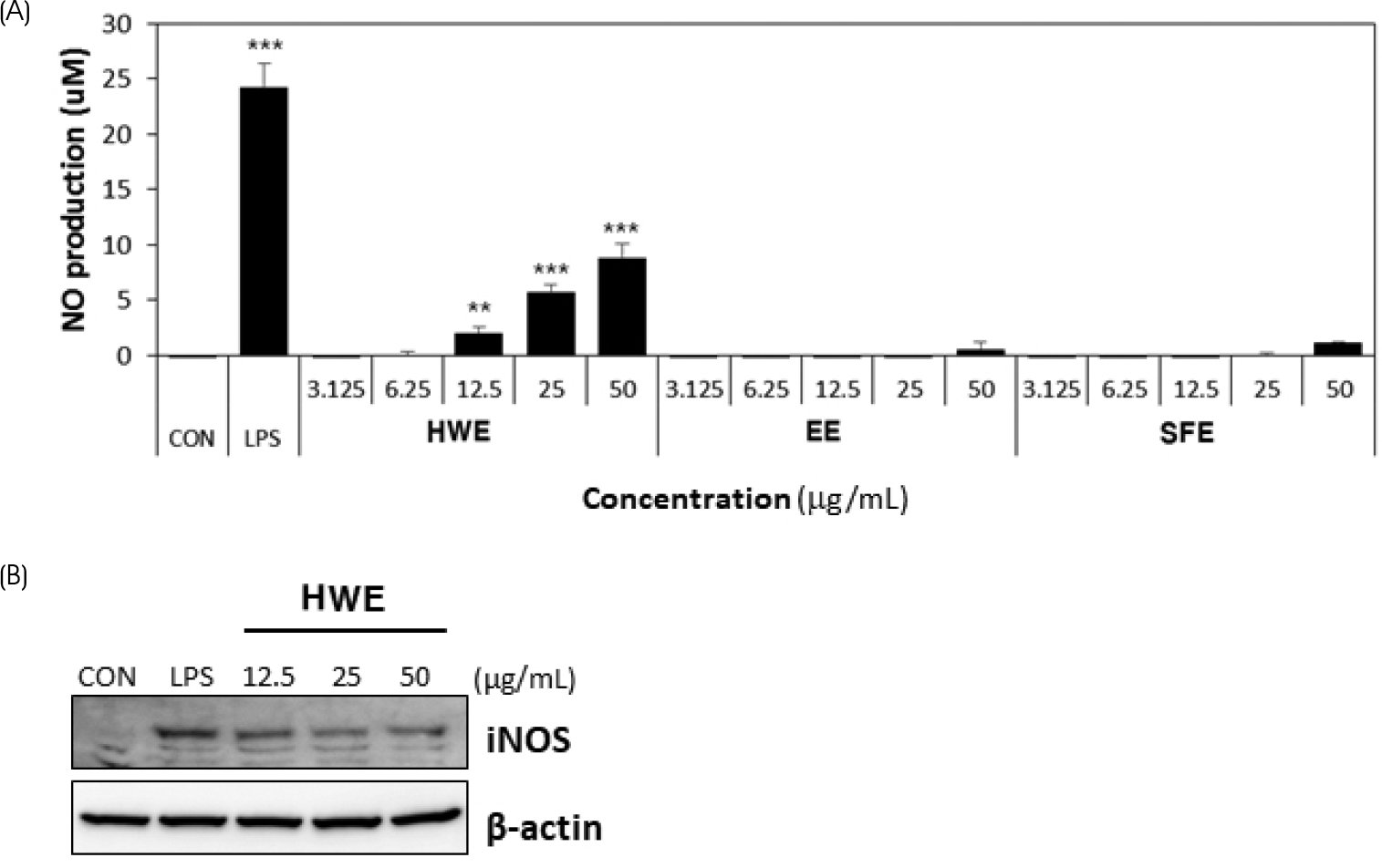 | Fig. 3.Effects of different types of Sargassum horneri extracts on the production of NO (A) and iNOS expression level (B) in Raw264.7 macrophages. (A) Dose-dependent effect of HWE from Sargassum horneri (3.125, 6.25, 12.5, 25, and 50 μg/mL) on NO production was determined by Griess reagent assay. LPS (1 μg/mL) used as a positive control. (B) Effect of HWE (12.5, 25, and 50 μg/mL) on iNOS expression in cell lysate was investigated by western blotting. Results are expressed as the mean ± SD (n = 3). Statistical analysis was performed using Student's two tails t-test with a significant level of ∗∗ p < 0.01, and ∗∗∗ p < 0.001, compared to control (CON) group. HWE, hot water extract; EE, ethanol extract; SFE, supercritical fluid extract |
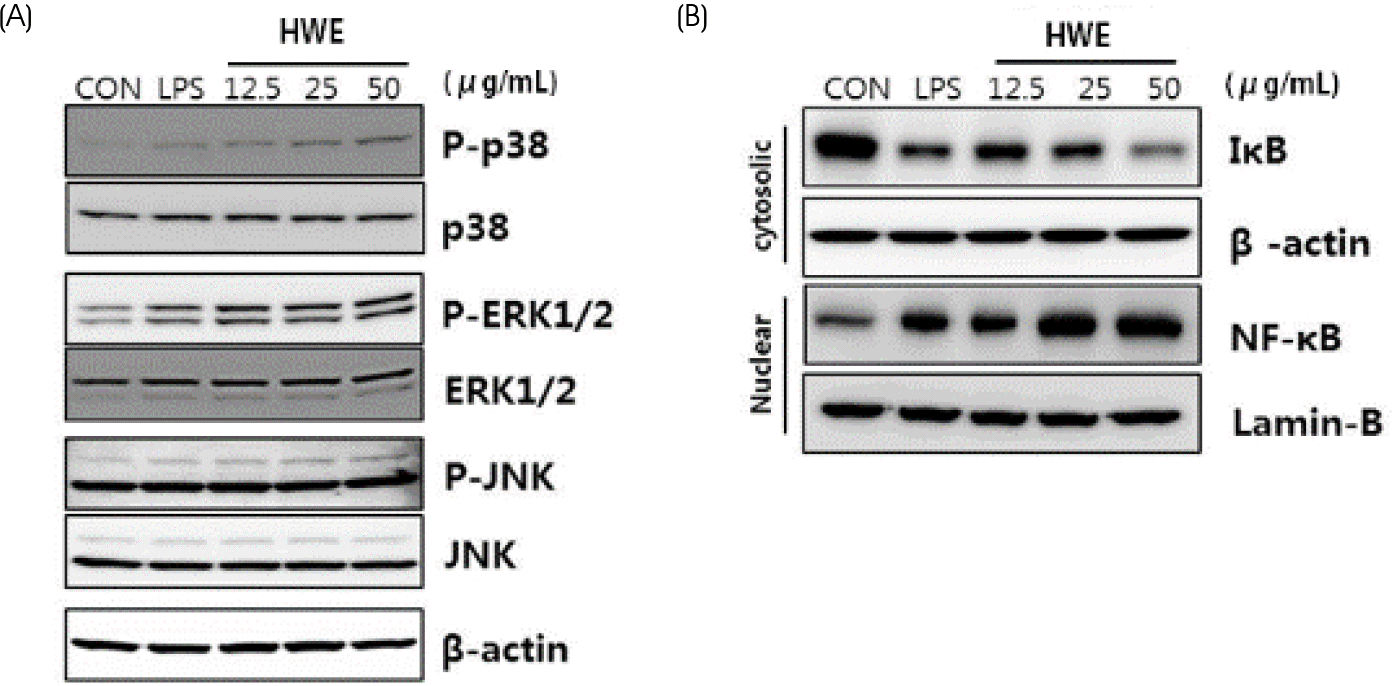 | Fig. 4.Effects of hot water extract of Sargassum horneri on MAPKs phosphorylation (A), cytosolic IκB and nuclear NF-κB expression (B) in RAW 264.7 macrophages. HWE of Sargassum horneri was treated at the concentration of 12.5, 25, and 50 μg/mL for 30 min. LPS (0.2 μg/mL) used as a positive control. MAPKs phosphorylation, IκB and NF-κB expression were evaluated by western bolt analysis using specific antibodies to p38, phospho-p38, ERK1/2, phospho-ERK1/2, JNK, phospho-JNK, IκB, NF-κB (p65). HWE, hot water extract |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download