Abstract
BACKGROUND/OBJECTIVES
MATERIALS/METHODS
RESULTS
Figures and Tables
 | Fig. 1 |
 | Fig. 2Revision process of the 2015 KDRIs. KNHANES, Korean National Health & Nutrition Examination Survey; EAR, estimated average requirement; RNI, recommended nutrient intake; UL, tolerable upper intake level; AI, adequate intake. Source: MOHW & KNS, 2015 [9]. |
Table 1
2015 KDRIs components for 1 year and older
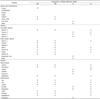
EAR, estimated average requirement; RNI, recommended nutrient intake; UL, tolerable upper intake level; AI, adequate intake
1) Estimated Energy Requirement (EER) for energy
2) Acceptable Macronutrient Distribution Range (AMDR) for energy sources among macronutrients
3) Recommendations for sugars
4) Intake goal
Source: Ministry of Health and Welfare (MOHW) & KNS, 2015 [9].
Table 2
Extracted data according to each study design

1,5) For review articles, AMSTAR [23] was used to assess the quality of studies.
2,5) For intervention studies, Jadad scale [20] was used to assess the quality of studies.
3,5) For cohort, nested case-control, and case-control studies, Newcastle-Ottawa [21] was used to assess the quality of studies.
4,5) For cross-sectional studies, STROBE [22] was used to assess the quality of studies.
Source: KNS, 2014 [27] .
Table 3
Number of studies reviewed for the 2015 KDRIs

1) Numbers in parentheses indicate the number of intervention studies, cohort studies, nested case-control studies, case-control studies, and cross-sectional studies in order.
2) Review of molybdenum study was not available due to the lack of study published after 2008.
Source: KNS, 2015 [28].
Table 4
Results of the quality assessment of studies used for the 2015 KDRIs

1) For intervention studies, Jadad scale [20] was used to assess the quality of studies.
2) For cohort, nested case-control, and case-control studies, Newcastle-Ottawa [21] was used to assess the quality of studies.
3) For cross-sectional studies, STROBE [22] was used to assess the quality of studies.
4) For review articles, AMSTAR [23] was used to assess the quality of studies.
5) Data presents average points and range (minimum to maximum points).
6) Molybdenum study was not included in the assessment due to the lack of study published after 2008.
Source: KNS, 2015 [28].
Notes
We would like to thank the chairs and members of the 2015 KDRIs review committee, and the 6 working subcommittees (age and physique, infants, energy & macronutrients, vitamins, minerals, and applications), for their tremendous efforts for establishing the 2015 KDRI. This project was funded by the Korean Ministry of Health and Welfare (Contract No. 2013: 12138153800; 2014: 25143119100; 2015: 25153092900).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download