Abstract
Purpose
Zinc (Zn) is an essential trace element for bone mineralization and osteoblast function. We examined the effects of Zn deficiency on osteoblast differentiation and mineralization in MC3T3-E1 cells.
Methods
Osteoblastic MC3T3-E1 cells were cultured at concentration of 1 to 15 µM ZnCl2 (Zn− or Zn+) for 5, 15 and 25 days up to the calcification period. Extracellular matrix mineralization was detected by staining Ca and P deposits using Alizarin Red and von Kossa stain respectively, and alkaline phosphatase (ALP) activity was detected by ALP staining and colorimetric method.
Results
Extracellular matrix mineralization was decreased in Zn deficiency over 5, 15, and 25 days. Similarly, staining of ALP activity as the sign of an osteoblast differentiation, was also decreased by Zn deficiency over the same period. Interestingly, the gene expression of bone-related markers (ALP, PTHR; parathyroid hormone receptor, OPN; osteopontin, OC; osteocalcin and COLI; collagen type I), and bone-specific transcription factor Runx2 were downregulated by Zn deficiency for 5 or 15 days, however, this was restored at 25 days.
In normal bone remodeling or bone turnover, osteoblastic bone formation and resorption is coupled in a precise and orchestrated manner. Osteoblastic differentiation is an essential aspect of bone formation. Several groups of proteins are necessary for osteoblastic differentiation, such as alkaline phosphatase (ALP) and transcription factors such as Runx2 etc.12
The trace element zinc (Zn) is an important regulator that plays many critical biological roles in all tissues and is a constituent of various enzymes and proteins.3 Zn deficiency is involved in bone retardation and malfunctions,45 as well as in the impairment of immune responses and brain functions.67 In humans, low Zn intake has been reported to be associated with low bone mass and weight loss in both children8 and adult women.9 In animal studies, Zn deficient diet decreased fetal long bone growth through decreased bone matrix formation in mice.10 Inadequate dietary Zn intake decreased the number of osteoblasts, and in vitro studies Zn dose-dependently augments DNA synthesis in murine osteoblast-like cells.11 In our studies, it has been reported that Zn stimulates osteoblast proliferation, differentiation through modulating osteoblast-specific gene expression and inorganic phosphate-producing enzyme alkaline phosphate in osteoblastic MC3T3-E1 cells.12131415
In this study, we further investigated whether cellular Zn deficiency affects osteoblast calcification in extending up to the extracellular matrix mineralization period, and also examined bone marker gene expression for osteoblast differentiation. We tested whether Zn deficiency decreased extracellular matrix mineralization measured by staining of Ca and P deposition as well as ALP enzyme activity which was measured by staining of the products of enzyme activity. We also demonstrated whether Zn deficiency decreased the expression of bone-related marker mRNA expression such as ALP, PTHR (parathyroid hormone receptor), OPN (osteopontin), OC (osteocalcin) and COLI (collagen type I), as well as bone-specific transcription factor, Runx2, in osteoblastic MC3T3-E1 cells.
The osteoblastic MC3T3-E1 cells were cultured at 5% CO2 at 37℃ in regular growth medium (α-MEM with 10% FBS, 1 mM sodium pyruvate, and 1% penicillin). For the differentiation of cells, 10 mM β-glycerol-2-phosphate and 50 µg/mL ascorbic acid were added to regular growth medium as previous described.14 Zn depletion was achieved by adding Zn chelator (N,N,N′,N′-tetrakis-(2-pyridylmethyl)-ethylenediamine [TPEN], cell membrane-permeable chelator, 5 µM), and the designated Zn levels for Zn deficiency (Zn−, 1 µM) and Zn adequacy (Zn+, 15 µM) were adjusted by externally adding ZnCl2 as previous described.14 We used the osteogenic differentiation medium without any Zn and TPEN treatment as the normal osteogenic control (osteogenic medium, OSM). Cells were cultured up to 5d, 15d (osteoblast differentiation period), and 25 days (mineralization period), and medium was changed every three days. Cellular zinc treatment and other experimental analysis were followed as previously mentioned.1216
The mineralized nodules in osteoblasts which were measured by Ca and P (as phosphate) deposition were visualized by staining analysis. MC3T3-E1 cells were stained with Alizarin Red S for the detection of extracellular matrix mineralization, combining with Ca of this dye for the detection of Ca deposition as recently described.14 For staining, MC3T3-E1 cells were washed with one volume of PBS and fixed with 4% formaldehyde in PBS for 15 min. Cells were then stained with 40 mM Alizarin Red S solution (pH 4.2) for 40 mins in the dark at room temperature. The appearance of red is an indication of the reaction between calcium ions and Alizarin red dye. The culture plates were photographed using a light microscope.
P deposition which is the nuclear initiation for forming bone nodules (extracellular P) was observed by the von Kossa staining as previously described.14 The cells were fixed with 4% formaldehyde in PBS for 15 min. The 5% silver nitrate solution was added to culture dishes, which were incubated under UV light for 60 mins. The culture plates were photographed using a light microscope.
Both ALP staining and enzyme activity assay were performed as previously described.14 Cellular ALP activity in the cells was normalized by total protein content of the cell lysate.
The cell lysates were diluted with deionized water. Concentrations of Zn, Ca, and P were measured by inductively coupled plasma-atomic emission spectrometry (ICP-AES; Flame Modula S, Spectro, Germany). The total protein content in the diluted cell lysates was also measured by BCA method. Cellular Zn, Ca and P contents were normalized by the total protein content of the cell lysate.
The transcription levels for ALP, PTHR, OC, OP, COLI, and Runx2 were determined by RT-PCR analysis as previously described.16 The Guanidinium thiocyanate method using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA) was used for the extraction of RNA from cell lysates, according to the manufacturer's instructions. RNA samples were reverse transcribed and amplified with PCR using standard protocols. The primers used for amplification of ALP, PTHR, OC, OP, COLI, Runx2, and GAPDH were as follows. ALP (204 bp): forward 5′-GCT GAT CAT TCC CAG GTT TT-3′; reverse 5′-CTG GGC CTG GTA GTT GTT GT-3′. PTHR (204 bp): forward 5′-GGG CAC AAG AAG TGG ATC AT-3′; reverse 5′-GGC CAT GAA GAC GGT GTA GT-3′. OC (219 bp): forward 5′-AAG CAG GAG GGC AAT AAG GT-3′; reverse 5′-TTT GTA GGC GGT CTT CAA GC-3′. OP (186 bp): forward 5′-TGC ACC CAG ATC TAG CC-3′; reverse 5′-CTC CAT CGT CAT CAT CAT CG-3′. COLI (170 bp): forward 5′-ACG TCC TGG TGA AGT TGG TC-3′; reverse 5′-CAG GGA AGC CTC TTT CTC CT-3′. Runx2 (90 bp): forward 5′-CGA GTC ATT TAA GGC TGC AA-3′; reverse 5′-AGG CTG TTT GAC GCC ATA GT-3′. GAPDH (200 bp): forward 5′-TCC ACT CAC GGC AAA TTC AAC G-3′; reverse 5′-TAG ACT CCA CGA CAT ACT CAG C-3′. The PCR products were electrophoresed on 1.2% agarose gel. The gels were then stained with ethidium bromide and photographed on a UV transilluminator.
Data were analyzed using SPSS 17.0 program (SPSS Inc., Chicago, IL, USA), and mean differences were considered as significant in cases that p < 0.05. Statistical analysis of the data was performed by one-way ANOVA to test the effect of different Zn levels. Once significance was detected, Tukey's HSD test was used to compare differences between the groups as post hoc analysis. Values are represented as mean ± SEM.
First, to assess whether Zn deficiency (Zn−) decreases the mineralization of MC3T3-E1 cell cell cultures, cells were first stained by Alizarin Red S solution for Ca accumulation. Our data showed that Zn deficiency (Zn−, 1 µM) decreased the Ca deposits as well as the formation of mineralized bone-like nodules, compared to Zn adequate (Zn+, 15 µM) or normal osteogenic control (OSM) in a time-dependent manner over 15 and up to 25 days. (Fig. 1A). However, there was no change in Ca deposits for 5 days in MC3T3-E1 cells. Zn adequate (Zn+) induced more osteoblast mineralization nodules after 15 days in MC3T3-E1 cells than in Zn−.
Next, we tested the osteoblast nodule that could be visualized by von Kossa staining for P depositions. We found that Zn deficiency was decreased in mineralization nodules which is a black staining for P depositions (Fig. 1B). Similarly, Zn adequate (Zn+) increased the osteoblast mineralized nodules for 15 or 25 days of calcification period. The results showed that Zn deficiency further decreased osteoblast mineralization at the late stage of differentiation (15d) and calcification period (25d) in osteoblasts.
Alkaline phosphatase (ALP) is the key enzyme for osteoblast differentiation and bone calcification in osteoblasts, since this enzyme is a prominent indicator for osteoblast differentiation as well as a key molecule for producing inorganic phosphate as a nucleator for osteoblast mineralization. To determine whether Zn deficiency inhibits osteoblast differentiation as well as mineralization through this particular enzyme ALP activity, cells were examined using both measurement in cell layers enzyme activity by staining as well as cellular and extracellular (in medium) ALP activity. ALP activity measurement and staining data showed that Zn deficiency (Zn−) decreased ALP activity, compared to Zn-adequate (Zn+) or normal control (OSM) (Fig. 2A). Cellular and media ALP activity was also decreased in Zn deficiency (Zn−), while Zn adequate (Zn+) increased ALP activity in a dose- and time-dependent manner (Fig. 2B and 2C).
To determine whether Zn deficiency modulates bone-related markers (ALP, PTHR, OC, OPN, and COLI) and osteoblast-specific transcription factor Runx2 in osteoblastic MC3T3-E1 cells, gene expression was analyzed by RT-PCR. The mRNA expression of bone markers (ALP, PTHR, OC, OPN, and COLI) were down-regulated in Zn deficiency (Zn−) than in Zn adequate (Zn+) over 5 and 15 days of bone differentiation period as well as the initial stage of the mineralization period (Fig. 3). Similarly, Runx2 mRNA expression was also downregulated in Zn− at 5 and 15 days, compared to Zn+ (Fig. 3).
Interestingly, these downregulated mRNAs for osteoblast markers in Zn− were less affected by Zn deficiency over 25 days of mineralization period (Fig. 3), which showed the expression pattern was almost compensated and was caught-up in Zn. This may imply that Zn deficiency in osteoblast initially downregulates bone marker genes but as time goes by it retards, but could be compensated at late stage. Our results suggest that Zn deficiency inhibited the bone marker gene expression as well as bone specific transcription factor Runx2, and this retardation may lead to the decreased bone differentiation and mineralization in osteoblasts.
Finally, to confirm whether Zn deficiency (Zn−) decreases Ca and P contents which are the major two minerals for osteoblast calcification in osteoblasts, cells were measured using ICP-AES analysis for mineral contents. Cellular Zn deficiency was properly confirmed in a dose- and time-dependent manner by Zn treatment (Fig. 4A). Interestingly, Ca and P contents in cells were increased by the addition of Zn in a dose- and time-dependent manner (Fig. 4B and 4C), and this pattern is consistent with the pattern of osteoblast calcification as Ca and P deposition (Fig. 1) and ALP activity (Fig. 2). In contrast, Ca and P contents were markedly decreased by Zn deficiency (Zn−) during the osteoblast differentiation and mineralization periods (5 and 20 days). It is considered that Zn increased osteoblast mineralization by increasing cellular Ca and P contents in osteoblasts.
The present study was conducted to determine whether Zn deficiency affected osteoblast differentiation and mineralization in MC3T3-E1 cells, measuring osteoblast Ca and P deposition in cell layers and bone marker gene expression including Runx2. In order to achieve the purpose of this study, we evaluated 1) the extracellular matrix mineralization depending on the cellular Zn level by staining Ca and P deposits, 2) inorganic phosphate-producing enzyme ALP activity by staining the product of enzyme activity and cellular enzyme activity, and 3) measured bone marker gene expression as well as bone transcription factor Runx2 during osteoblast differentiation (15d) and mineralization period (25d). Our data showed that 1) osteoblast Zn deficiency decreased the transcription level of bone marker genes (ALP, OPN, OC, and COL1) as well as bone-specific transcription factor Runx2 in MC3T3-E1 cells, and this decreased gene expression by Zn deficiency was restored in the late stage of the mineralization period with a retarded pattern which was induced by Zn deficiency. 2) The decreased mineralization by Zn deficiency, which was confirmed by the decreased Ca and P deposition, might be related to the decreased osteoblast-specific gene expression by Zn deficiency.
The bone mineralization process has been clarified by in vivo and in vitro studies on matrix vesicles from cartilage and bone and mainly consists of two phases.17 Extracellular matrix production and extracellular environment in osteoblast have both been reported to be involved in the mineralization of osteoblasts.18 Enzyme ALP is also present in various other tissues in general and signals cellular communication, meanwhile osteoblastic ALP is a specific enzyme in osteoblasts for producing mainly inorganic phosphate. A key to understanding the role of ALP in bone mineralization is provided by studies of the phased expression of genes during osteoblastic differentiation and growth plate cartilage calcification.1920 In bone, ALP is expressed early in bone formation, and is soon observed on the cell surface and in matrix vesicles. Anderson et al. concluded that the early phase of mineralization was closely associated with tissue non-specific ALP.18 Matrix mineralization is initiated by the nucleation of Ca deposits, and this process is normally initiated by extracellular matrix vesicles, which are synthesized by osteoblasts, budded from the cell plasma membrane and released into the extracellular matrix. ALP is a component of these matrix vesicles along with some other Ca2+-dependent proteins and Ca2+-Piphospholipid complexes.21 According to our previous report, Zn adequacy increased ALP activity and Ca/P deposits as measured by Alizarin Red and von Kossa stains.1415 In the present study, Zn deficiency decreased ALP activity and Ca/P deposits over 5, 15, and 25 days which covers the full bone formation period. In addition, the increased Ca and P deposition may be partly due to the increased cellular ALP activity by Zn adequacy.
Runx2, a bone-specific transcription factor, is a key regulator of osteoblast differentiation and bone formation.22 Runx2-deficient mice show a complete lack of ossified bones, and thus Runx2 has been implicated as the master gene of osteoblast differentiation.23 Runx2 regulates the osteoblast gene expression for the proteins (including ALP, OPN, OC, and COLI) during osteoblast differentiation and mineralization period, and these genes and proteins are expressed by osteoblasts.22 In fact, OPN, ALP, and COLI mRNAs were expressed at the time of osteoblast differentiation. Osteocalcin (OC) is a later marker of osteoblast differentiation that is closely related to osteoblast maturation.24 Our results showed that bone-related marker mRNAs were downregulated by Zn deficiency during the osteoblast differentiation period (5d and 15d) and in late stage, the expressions of those bone marker genes were restored to catch up osteoblast differentiation and mineralization process. Consistently, the osteoblast differentiation transcription factor Runx2 expression was decreased by Zn deficiency during osteoblast differentiation period.
Also, our data showed that expression pattern of Runx2 and its regulatory bone marker genes such as ALP, PTHR, OPN, OC and COLI were less affected by Zn deficiency during late stage of osteoblast mineralization period (25d), compared to during the early stage of osteoblast proliferation and differentiation (5d and 15d). This pattern is unique as it implies that the down-regulated gene expression by Zn deficiency at early stage of bone formation period were only delayed and retarded, but could be gradually caught-up as the bone-forming process went on.
Finally, Zn deficiency also modulated decreasing Runx2 mRNA expression, and thus inhibiting the bone-related marker mRNAs expression, which can be a potential reason for the retarded osteoblast differentiation and mineralization by Zn deficiency in osteoblasts.
In conclusion, we have demonstrated that Zn deficiency decreased osteoblast differentiation and mineralization in osteoblastic MC3T3-E1 cells by decreasing Ca and P deposition, ALP activity, and bone marker gene expression. Therefore, our results showed that Zn deficiency in osteoblasts inhibited osteoblast differentiation and mineralization through inhibiting bone-marker gene expression (ALP; alkaline phosphatase, PTHR; parathyroid hormone receptor, OPN; osteopontin, OC; osteocalcin and COLI; collagen type I) and bone-specific transcription factor Runx2 expression. The decreased Ca and P deposition and the downregulated gene expression by Zn deficiency might be the major reason for poor osteoblast differentiation and mineralization under low cellular zinc status in osteoblasts.
Figures and Tables
 | Fig. 1Cellular Zn deprivation inhibited extracellular matrix (ECM) mineralization (Ca and P deposits) and bone nodule formation in osteoblastic MC3T3-E1 cells. (A–B) Cellular morphology of MC3T3-E1 cells which were treated with normal osteogenic control (OSM), Zn− (1 µM) and Zn+ (15 µM) for 5, 15, and 25 days. Analyzed by Alizarin Red for Ca deposits (A, red color) and von Kossa staining for P deposits (B, dark black color). Different superscripts mean significant differences between Zn treatments on the same treatment time at p < 0.05 by Tukey, ANOVA. |
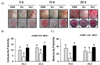 | Fig. 2Cellular Zn deprivation decreased the product of alkaline phosphatase activity expression and cellular and medium ALP activity in osteoblastic MC3T3-E1 cells. (A) The staining of the product of ALP activity in MT3T3-E1 cell layers treated with control (OSM), Zn− (1 µM) and Zn+ (15 µM) for 5, 15, and 25 days, analyzed by ALP staining for the products of enzyme activity (red color). (B and C) ALP enzyme activity of cells and media under control (OSM), Zn− (1 µM) and Zn+ (15 µM) conditions for 15 and 25 days. Values are mean ± SEM. Different superscripts mean significant differences between Zn treatments on the same treatment time at p < 0.05 by Tukey, ANOVA. Unit for cellular ALP activity (nmol para-nitrophenol/mg protein/min). Unit for medium ALP activity (nmol para-nitrophenol/mL/min). |
 | Fig. 3Cellular Zn deprivation downregulated bone marker and bone transcription factor Runx2 gene expression in osteoblastic MC3T3-E1 cells. mRNA transcription levels of bone markers treated with Zn− (1 µM) and Zn+ (15 µM) for 5, 15, and 25 days were measured using RT-PCR analysis. (ALP; alkaline phosphatase, PTHR; parathyroid hormone receptor, OPN; osteopontin, OC; osteocalcin and COLI; collagen type I). |
Notes
References
1. Banerjee C, Javed A, Choi JY, Green J, Rosen V, van Wijnen AJ, Stein JL, Lian JB, Stein GS. Differential regulation of the two principal Runx2/Cbfa1 n-terminal isoforms in response to bone morphogenetic protein-2 during development of the osteoblast phenotype. Endocrinology. 2001; 142(9):4026–4039.

2. Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002; 108(1):17–29.

3. Prasad AS. Zinc: an overview. Nutrition. 1995; 11:1 Suppl. 93–99.
4. Leek JC, Vogler JB, Gershwin ME, Golub MS, Hurley LS, Hendrickx AG. Studies of marginal zinc deprivation in rhesus monkeys. V. Fetal and infant skeletal effects. Am J Clin Nutr. 1984; 40(6):1203–1212.

5. Yamaguchi M. Role of nutritional zinc in the prevention of osteoporosis. Mol Cell Biochem. 2010; 338(1-2):241–254.

6. Chowanadisai W, Kelleher SL, Lönnerdal B. Maternal zinc deficiency reduces NMDA receptor expression in neonatal rat brain, which persists into early adulthood. J Neurochem. 2005; 94(2):510–519.

7. Zhao N, Wang X, Zhang Y, Gu Q, Huang F, Zheng W, Li Z. Gestational zinc deficiency impairs humoral and cellular immune responses to hepatitis B vaccination in offspring mice. PLoS One. 2013; 8(9):e73461.

8. Salgueiro MJ, Zubillaga MB, Lysionek AE, Caro RA, Weill R, Boccio JR. The role of zinc in the growth and development of children. Nutrition. 2002; 18(6):510–519.

9. Angus RM, Sambrook PN, Pocock NA, Eisman JA. Dietary intake and bone mineral density. Bone Miner. 1988; 4(3):265–277.
10. Kim JT, Baek SH, Lee SH, Park EK, Kim EC, Kwun IS, Shin HI. Zinc-deficient diet decreases fetal long bone growth through decreased bone matrix formation in mice. J Med Food. 2009; 12(1):118–123.

11. Yamaguchi M, Matsui T. Stimulatory effect of zinc-chelating dipeptide on deoxyribonucleic acid synthesis in osteoblastic MC3T3-E1 cells. Peptides. 1996; 17(7):1207–1211.

12. Alcantara EH, Lomeda RA, Feldmann J, Nixon GF, Beattie JH, Kwun IS. Zinc deprivation inhibits extracellular matrix calcification through decreased synthesis of matrix proteins in osteoblasts. Mol Nutr Food Res. 2011; 55(10):1552–1560.

13. Cho YE, Lomeda RA, Ryu SH, Sohn HY, Shin HI, Beattie JH, Kwun IS. Zinc deficiency negatively affects alkaline phosphatase and the concentration of Ca, Mg and P in rats. Nutr Res Pract. 2007; 1(2):113–119.

14. Cho YE, Kwun IS. Zinc upregulates bone-specific transcription factor Runx2 expression via BMP-2 signaling and Smad-1 phosphorylation in osteoblasts. J Nutr Health. 2018; 51(1):23–30.

15. Seo HJ, Cho YE, Kim T, Shin HI, Kwun IS. Zinc may increase bone formation through stimulating cell proliferation, alkaline phosphatase activity and collagen synthesis in osteoblastic MC3T3-E1 cells. Nutr Res Pract. 2010; 4(5):356–361.

16. Kwun IS, Cho YE, Lomeda RA, Shin HI, Choi JY, Kang YH, Beattie JH. Zinc deficiency suppresses matrix mineralization and retards osteogenesis transiently with catch-up possibly through Runx 2 modulation. Bone. 2010; 46(3):732–741.

18. Anderson HC, Sipe JB, Hessle L, Dhanyamraju R, Atti E, Camacho NP, Millán JL. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am J Pathol. 2004; 164(3):841–847.

19. Golub EE, Harrison G, Taylor AG, Camper S, Shapiro IM. The role of alkaline phosphatase in cartilage mineralization. Bone Miner. 1992; 17(2):273–278.

20. Orimo H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J Nippon Med Sch. 2010; 77(1):4–12.

22. Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997; 89(5):747–754.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download