Abstract
Purpose
The use of oncoplastic reconstruction for breast-conserving surgery (BCS) extends benefits beyond merely minimizing poor cosmetic results. However, the feasibility and oncological safety of oncoplastic surgery (OPS) are controversial.
Methods
This meta-analysis aimed to compare the short-term and long-term oncological outcomes of BCS alone and BCS plus OPS. Relevant studies published before July 2017 in the Embase, the Cochrane Library, PubMed, and Web of Science databases were screened and collected. The meta-analysis was performed using STATA software (Stata Corp.).
Results
A total of 3,789 patients from 11 studies were included, with 2,691 patients in the BCS-alone group and 1,098 patients in the BCS plus OPS group. The demographics were similar between both groups, and no significant difference was observed in pathological T and N stages between the two groups. Re-excision was less common (relative risk [RR], 0.66; p=0.009) and the positive-margin rate was lower, but not significantly (RR, 0.83; p=0.191), in the BCS plus OPS group than in the BCS-alone group. The local and distal recurrence rates were similar in both groups. Both disease-free survival (hazard ratio [HR], 1.19; 95% confidence interval [CI], 0.96–1.49; p=0.112) and overall survival (HR, 1.14; 95% CI, 0.76–1.69; p=0.527) did not differ between the two groups.
Breast-conserving surgery (BCS) is oncologically and surgically safe, with a long-term survival comparable to total mastectomy [12]. However, the aesthetic outcomes of breast shape and symmetry resulting from BCS are not always optimal. In 25%–30% of women, the aesthetic outcome is compromised after BCS [3]. In addition, BCS is not suitable under certain conditions such as large or multicentric tumors and in cases involving the skin or pectoral muscle. Moreover, with BCS, free margins cannot be obtained in specific oncologic and anatomic conditions such as tumors larger than 5 cm in diameter, high tumor-to-breast ratio, or multicentric tumors [4]. Previous studies reported that positive margins were found in 10%–40% of the cases, with re-excision rates up to 60% in BCS cases [56]. Such high incidences of re-excision result in high morbidity, complications, and healthcare costs [78].
A combination of oncoplastic surgery (OPS) and BCS has been developed to overcome the abovementioned shortcomings. The primary aim of OPS is to reduce deformity and attain acceptable breast appearance. Many oncoplastic breast-conserving techniques have been previously developed. In general, the technical approaches can be categorized into three main types: breast-reduction techniques, volume-replacement techniques, and en-bloc closure techniques for breast defects [9]. Because OPS involves reshaping of the whole breast, it allows wider excision of the tumor, thus increasing the likelihood of performing conservative surgery for patients previously considered unsuitable for BCS, including those with a large tumor, unfavorable tumor-to-breast ratio, central and low-pole tumor location, or multifocal disease [10]. However, outcomes of other oncologic parameters such as positive margins and cancer recurrence have not yet been analyzed for OPS.
Therefore, we performed a meta-analysis to compare BCS alone and BCS plus OPS to determine the oncological outcomes of BCS plus OPS. With a large sample size, it is possible to eliminate some of the inherent selection bias associated with these procedures.
Several databases including PubMed, Web of Science, Embase, and the Cochrane Library were searched by two independent reviewers by using the following terms: (1) oncoplastic OR conserving OR segmental OR segmentectomy OR lumpectomy OR local excision OR mastectomy AND (2) breast AND (3) neoplasm OR cancer OR tumor OR carcinoma OR malignant AND (4) recurrence OR relapse AND (5) survival. Reviews of interest were also retrieved from the reference lists of relevant studies. Only studies published before July 2017 were included in the search.
The following studies were selected in the meta-analysis: (1) studies including at least two groups of patients with OPS or BCS; (2) studies comparing short-term outcomes of OPS and BCS including margin status, re-excision, tumor size, and pathological T and N stages; (3) studies comparing long-term outcomes of OPS and BCS including local, regional, and distal relapse, disease-free survival (DFS), and overall survival (OS); and (4) studies describing the clinicopathological characteristics of patients.
The exclusion criteria were as follows: (1) OPS with oncoplastic mastectomy; (2) review, case report, conference abstract, and comment articles; (3) experimental studies based on non-human subjects; and (4) duplicate studies or data from the same patient cohort.
Data collection from all included studies was performed by two independent investigators. The following general and clinicopathological characteristics were extracted from the eligible studies: first author's names, publication year, country, study period, number of patients, TNM stage, age, gender, diagnosis (invasive, in situ, and others), tumor characteristics, specimen weight and size, margin status, re-excision rate, length of follow-up, and outcomes (survival and recurrence). Discrepancies in the data were further discussed, and a consensus was reached for all extracted parameters.
Pooled analysis was performed using STATA version 12 software (Stata Corp., College Station, USA). The I2 statistic was used to measure the statistical heterogeneity in the meta-analysis of each clinicopathological parameter. The fixed-effects model (the Mantel-Haenszel method) was used if the heterogeneity was accepted, and the random-effects model (the DerSimonian-Laird method) was used if there was considerable statistical heterogeneity. The Begg's and Egger's tests were performed to calculate the publication bias. Values of p<0.05 were defined as statistically significant in all pooled comparisons.
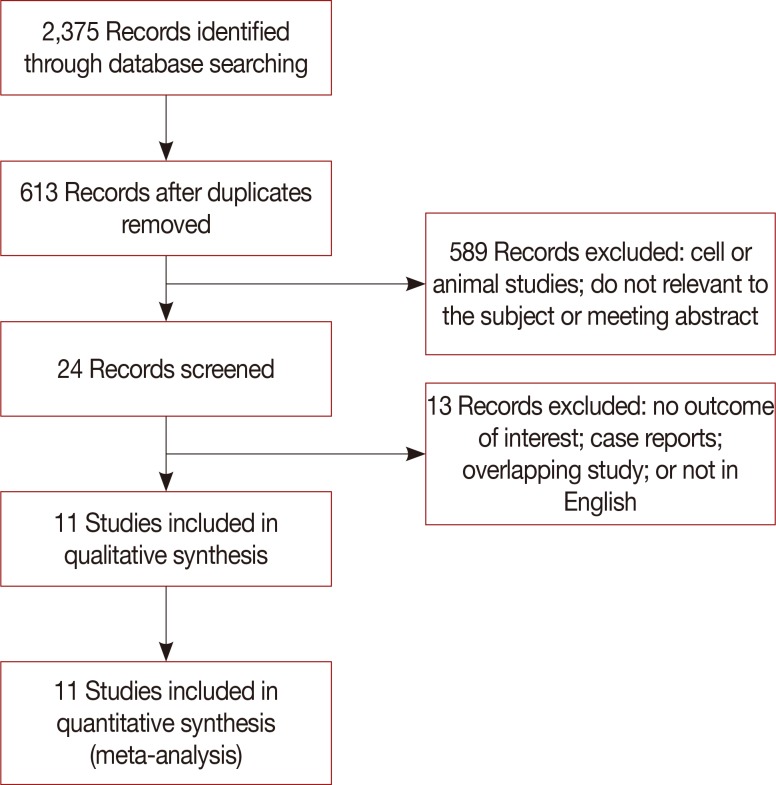
A total of 2,375 studies were initially selected from the literature. Of these, 11 studies [1011121314151617181920] were finally included in the meta-analysis. The excluded studies were duplicated studies from different websites, studies with overlapping data, case reports, conference abstracts, and studies based on experimental or non-human subjects (Figure 1). A total of 3,789 cases (2,691 patients in the BCS group and 1,098 in the BCS plus OPS group) from the 11 studies were included in the meta-analysis (Table 1).
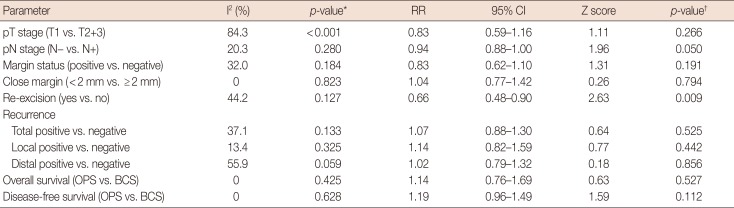
The OPS techniques used in the included studies were glandular or pedicle flap transfer, wise-pattern procedure, Grisotti procedure, Benelli procedure, donut mastopexy, reduction mammoplasty following lumpectomy, quadrantectomy, or wide local excision. Young patients with breast cancer tended to choose OPS; the average age of patients in the OPS group was lower in seven studies [10121316171819], of which three studies [131819] reached statistical significance. All the included studies compared the oncological outcomes of BCS and BCS plus OPS for breast cancer, and the major histopathological type was ductal carcinoma (83.8%–71.6%). There was no significant difference in the in situ (BCS vs. OPS, 5.1%–18.6% vs. 8.9%–19.4%, respectively) and invasive disease (BCS vs. OPS, 74.4%–85.6% vs. 74.2%–91.3%) diagnoses between the BCS and BCS plus OPS groups. The random-effects model used to determine the pooled relative risk (RR) of pathological T stages due to statistical heterogeneity (I2=84.3%, p<0.001) showed no difference in the pathological T stage (T1 vs. T2+T3; RR, 0.83; 95% confidence interval [CI], 0.59–1.16; p=0.266) (Table 2, Figure 2A); in contrast, the pathological N stage (N0 vs. N1; RR, 0.94; 95% CI, 0.88–1.00; p=0.050) was marginally significant in the BCS-alone and BCS plus OPS groups (Table 2, Figure 2B).
Positive-margin and re-excision rates are the pivotal short-term factors associated with the safety and feasibility of surgical techniques. To evaluate the impact of OPS plus BCS, we analyzed seven of the included studies that reported the margin status during BCS and OPS procedures. In the meta-analysis, neither the positive-margin rate (RR, 0.83; 95% CI, 0.62–1.10; p=0.191) nor the close-margin rate (RR, 1.04; 95% CI, 0.77–1.42; p=0.794) showed any difference between the BCS-alone and BCS plus OPS groups (Table 2, Figure 3). In contrast, the re-excision rate was significantly lower in the BCS plus OPS group (RR, 0.66; 95% CI, 0.48–0.90; p=0.009) (Table 2, Figure 4), which indicated a better therapeutic effect of BCS plus OPS than BCS alone. The Begg's and Egger's tests showed no statistically significant publication bias (p>0.05, data not shown).
To assess the long-term differences between BCS alone and BCS plus OPS, the recurrence and survival data were collected from the included studies. Nine studies reported the recurrence rate following BCS plus OPS and BCS. The pooled data showed that the total relapse rate was similar in the two groups (RR, 1.07; 95% CI, 0.88–1.30; p=0.525). Furthermore, the results were close for both the local (RR, 1.14; 95% CI, 0.82–1.59; p=0.442) and distal recurrence rates (RR, 1.02; 95% CI, 0.79–1.32; p=0.856) (Table 2, Figure 5). The Begg's and Egger's tests revealed no statistically significant publication bias (p>0.05, data not shown).
The survival data also revealed non-inferior effects of OPS plus BCS compared with BCS alone. The meta-analysis examined the 10-year OS and DFS with BCS alone and BCS plus OPS, and the results showed that BCS alone and BCS plus OPS had similar long-term effectiveness in terms of the OS (hazard ratio [HR], 1.14; 95% CI, 0.76–1.69; p=0.527) and DFS (HR, 1.19; 95% CI, 0.96–1.49; p=0.112) after the procedures (Table 2, Figure 6).
The unsatisfactory cosmetic results after BCS has tremendously increased the popularity of OPS. OPS can prevent deformities and unsatisfactory aesthetic effects resulting from BCS [49]. Two main factors that impact the aesthetic results are tumor-to-breast volume and tumor location. A volume excision of 10% is usually considered an aesthetically acceptable limit for BCS. Due to the relative tissue paucity, a reduction of >5% medially can lead to bad aesthetic results, but it is possible to remove up to 15% of the breast volume laterally with a positive outcome. Our meta-analysis showed that OPS allows wide excisions with free margins and can be used for large cancers as an alternative to mastectomy. Additionally, OPS did not affect long-term survival in patients. Owing to these factors, OPS has more advantages over BCS.
In the plastic surgery community, identifying the indications of OPS is important. However, the suitability for immediate or delayed OPS has remained a controversy. The National Comprehensive Cancer Network guidelines for breast cancer (version 2, 2017) suggest that reconstruction should not interfere with appropriate surgical management of cancer or the scope of appropriate surgical treatment for the disease. Surgical options for reconstruction include breast implants, autologous tissue transplantation, and a combination of the two methods. According to the reports included in our study, OPS was largely determined by the tumor-to-breast ratio, tumor location, and patient anatomy and preferences. Decisions on when and how to perform OPS vary by surgeons; therefore, the outcome of this meta-analysis could serve as evidence for appropriate decision making with regard to OPS in clinical practice.
OPS is suitable for different breast sizes and shapes, not just large breasts. Although it is not easy to use OPS for small breasts or in patients with high tumor-to-breast ratio, personalized OPS can be designed with the variety of available OPS surgical techniques and timings. Plastic surgery consultations are necessary for patients with small breasts who experience borderline cosmetic results after BCS. It has been reported that volume-replacement techniques such as latissimus dorsi myocutaneous flap transfer have advantages over volume-displacement techniques for such patients [21], even when 50% of the breast volume is resected [22]. In addition, if the cosmetic result is suboptimal, a second oncoplastic surgery can be performed to compensate for the aesthetic defects.
Although OPS benefits patients by reducing the re-excision rate, our pooled results did not show any advantage of OPS in terms of reducing the positive-margin rate. Re-excision of positive margins after initial lumpectomy for breast cancer is recommended by the guidelines and represents quality care [23]. However, re-excision in breast cancer surgery is important for reducing positive margins and close margins (<1 mm or <2 mm) [24]. Due to the inconclusive indications for reexcision, a different surgical team may use re-excision for various reasons such as an ink-positive margin, uncertain margin status, fragmented specimen, and evidence of residual disease found by postoperative imaging. Thus, the re-excision rate may be higher than the positive-margin rate. Correspondingly, the outcome of our meta-analysis reveals a decreasing trend in the positive-margin rate but a significantly lower reexcision rate with OPS. The reduced re-excision rate in the OPS group did not translate to low recurrence rates or long survival time, which also indicates that re-excision is not a remarkable prognostic factor after breast cancer surgery.
Although the ability to obtain a generous resection by OPS while maintaining the breast shape is an obvious advantage of OPS, its oncological advantages are controversial. Negative surgical margins should be obtained after removing the breast cancer. For tumors with similar sizes, a wide resection is more likely to involve normal adjacent tissue and yield a low positive-margin rate. Sometimes, positive margins merely occur because of the radiated nature of the tumor, which is why reexcisions often do not increase the positive-margin rates; however, wide resections add to the chances of obtaining free margins.
One limitation of this meta-analysis is that subgroup analysis is usually restricted by the results of the included studies. There are three main approaches of OPS: (1) reconstruction with implants and skin expanders, vascularized tissue flaps, nonvascularized lipoaspirate fat; (2) local tissue rearrangement, mastopexy; and (3) reduction mammoplasty [25]. However, we could not perform a meta-analysis analyzing different OPS methods because the methods were not compared in all the included studies. The prognostic impacts of those OPS methods seemed similar due to their common characteristic of better control of free margins as compared to BCS alone. To the best of our knowledge, no study has thus far reported different associations of OPS methods with tumor recurrence or patient survival. Therefore, we believe that the outcome of our meta-analysis is reliable, despite the use of different methods in the OPS group.
The similarity distribution of pathological T and N stages in the BCS-alone and BCS plus OPS groups strengthen the reliability of our results. However, the pathological N stage occurred notably earlier in the BCS plus OPS group, and the difference was marginally significant. It is possible that patients with early stage breast cancer without suspicious positive axillary lymph nodes would tend to choose OPS. The lymph node metastasis status is the most-important prognostic factor in breast cancer [26]. Thus, the earlier nodal stage might explain the trend of better survival in the BCS plus OPS group. In future prospective clinical trials or real-world studies, the randomized control or propensity score-matched strategy will help reduce patient-selection bias.
The expression levels of hormone receptors [27] or cell mitosis factors [28] are associated with long-term survival in patients with breast cancer. Although lacking patient data on pathological molecular status such as, estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2, Ki-67 antigen, or proliferating cell nuclear antigen might add biases to the survival outcomes, we believe that patients with various molecular types of cancer were randomly distributed in the BCS alone and BCS plus OPS groups, because the expression of those factors was usually unknown before surgery and was examined from the postoperative specimens. Therefore, we analyzed the molecular expression in the included cases; the outcome demonstrated no difference between the BCS-alone and BCS plus OPS groups (Supplementary Figure 1, available online). Adjuvant radiotherapy, endocrine therapy, and chemotherapy administered on the basis of the molecular types of cancer also influenced the long-term survival outcome [29]. However, the proportion of patients who underwent the adjuvant treatment was similar in both surgery groups (Supplementary Figure 2, available online), thus, the consistency in the molecular status and adjuvant therapy enhanced the reliability of the outcomes of our meta-analysis.
To our knowledge, our results is the first to provide effective evidence from a meta-analysis comparing BCS alone and BCS plus OPS. Although Losken et al. [30] previously discussed the issue, their study was a pooled retrospective re-analysis of separated OPS or BCS cohort data, rather than a systematic review and meta-analysis. Therefore, the RR and HR differences were calculated using the chi-square test instead of the Mantel-Haenszel method. Moreover, their comparison of survival with different follow-up lengths made it difficult to obtain accurate results. In contrast, we performed this meta-analysis with proper methods and chose 5 years after surgery as the cutoff time for survival and relapse calculations.
Owing to the nature of meta-analyses, our study had a several limitations. First, all related clinicopathological parameters such as re-excision rates and positive margins could not be analyzed in the same patient cohort due to limited original data from the included studies. Second, studies with a large sample size were included from different countries across Europe, America, and Asia, which increased the accuracy of comparisons but introduced heterogeneity. Therefore, some of the pooled comparisons were performed using the random-effects model, and these results should be interpreted carefully. Nevertheless, the purpose of the meta-analysis was not to confirm the hypotheses, but to provide suggestions and insights for future studies to provide conclusive recommendations on the hypotheses.
In conclusion, OPS could serve as a valuable technique for patients with breast cancer. Our meta-analysis showed that the OPS techniques have benefits extending beyond minimization of poor cosmetic outcomes. Future studies with new cohorts and long follow-ups are necessary to confirm our findings.
References
1. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002; 347:1233–1241. PMID: 12393820.

2. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002; 347:1227–1232. PMID: 12393819.

3. Clough KB, Cuminet J, Fitoussi A, Nos C, Mosseri V. Cosmetic sequelae after conservative treatment for breast cancer: classification and results of surgical correction. Ann Plast Surg. 1998; 41:471–481. PMID: 9827948.

4. Clough KB, Benyahi D, Nos C, Charles C, Sarfati I. Oncoplastic surgery: pushing the limits of breast-conserving surgery. Breast J. 2015; 21:140–146. PMID: 25676776.

5. Wanis ML, Wong JA, Rodriguez S, Wong JM, Jabo B, Ashok A, et al. Rate of re-excision after breast-conserving surgery for invasive lobular carcinoma. Am Surg. 2013; 79:1119–1122. PMID: 24160812.

6. Biglia N, Ponzone R, Bounous VE, Mariani LL, Maggiorotto F, Benevelli C, et al. Role of re-excision for positive and close resection margins in patients treated with breast-conserving surgery. Breast. 2014; 23:870–875. PMID: 25305040.

7. Jung W, Kang E, Kim SM, Kim D, Hwang Y, Sun Y, et al. Factors associated with re-excision after breast-conserving surgery for early-stage breast cancer. J Breast Cancer. 2012; 15:412–419. PMID: 23346170.

8. Swanson GP, Rynearson K, Symmonds R. Significance of margins of excision on breast cancer recurrence. Am J Clin Oncol. 2002; 25:438–441. PMID: 12393979.

9. Macmillan RD, McCulley SJ. Oncoplastic breast surgery: what, when and for whom? Curr Breast Cancer Rep. 2016; 8:112–117. PMID: 27330677.

10. Chakravorty A, Shrestha AK, Sanmugalingam N, Rapisarda F, Roche N, Querci Della Rovere G, et al. How safe is oncoplastic breast conservation? Comparative analysis with standard breast conserving surgery. Eur J Surg Oncol. 2012; 38:395–398. PMID: 22436560.

11. De Lorenzi F, Hubner G, Rotmensz N, Bagnardi V, Loschi P, Maisonneuve P, et al. Oncological results of oncoplastic breast-conserving surgery: long term follow-up of a large series at a single institution: a matched-cohort analysis. Eur J Surg Oncol. 2016; 42:71–77. PMID: 26382101.
12. Calì Cassi L, Vanni G, Petrella G, Orsaria P, Pistolese C, Lo Russo G, et al. Comparative study of oncoplastic versus non-oncoplastic breast conserving surgery in a group of 211 breast cancer patients. Eur Rev Med Pharmacol Sci. 2016; 20:2950–2954. PMID: 27460720.
13. Mansell J, Weiler-Mithoff E, Stallard S, Doughty JC, Mallon E, Romics L. Oncoplastic breast conservation surgery is oncologically safe when compared to wide local excision and mastectomy. Breast. 2017; 32:179–185. PMID: 28214785.

14. Vieira RA, Carrara GF, Scapulatempo Neto C, Morini MA, Brentani MM, Folgueira MA. The role of oncoplastic breast conserving treatment for locally advanced breast tumors: a matching case-control study. Ann Med Surg (Lond). 2016; 10:61–68. PMID: 27547399.

15. Gulcelik MA, Dogan L, Yuksel M, Camlibel M, Ozaslan C, Reis E. Comparison of outcomes of standard and oncoplastic breast-conserving surgery. J Breast Cancer. 2013; 16:193–197. PMID: 23843852.

16. Chauhan A, Sharma MM. Evaluation of surgical outcomes following oncoplastic breast surgery in early breast cancer and comparison with conventional breast conservation surgery. Med J Armed Forces India. 2016; 72:12–18. PMID: 26900217.

17. Giacalone PL, Roger P, Dubon O, El Gareh N, Rihaoui S, Taourel P, et al. Comparative study of the accuracy of breast resection in oncoplastic surgery and quadrantectomy in breast cancer. Ann Surg Oncol. 2007; 14:605–614. PMID: 17151794.

18. Kaur N, Petit JY, Rietjens M, Maffini F, Luini A, Gatti G, et al. Comparative study of surgical margins in oncoplastic surgery and quadrantectomy in breast cancer. Ann Surg Oncol. 2005; 12:539–545. PMID: 15889210.

19. Tenofsky PL, Dowell P, Topalovski T, Helmer SD. Surgical, oncologic, and cosmetic differences between oncoplastic and nononcoplastic breast conserving surgery in breast cancer patients. Am J Surg. 2014; 207:398–402. PMID: 24581764.

20. Mazouni C, Naveau A, Kane A, Dunant A, Garbay JR, Leymarie N, et al. The role of oncoplastic breast surgery in the management of breast cancer treated with primary chemotherapy. Breast. 2013; 22:1189–1193. PMID: 24054903.

21. Yang JD, Lee JW, Cho YK, Kim WW, Hwang SO, Jung JH, et al. Surgical techniques for personalized oncoplastic surgery in breast cancer patients with small- to moderate-sized breasts (part 1): volume displacement. J Breast Cancer. 2012; 15:1–6. PMID: 22493622.

22. Franceschini G, Terribile D, Magno S, Fabbri C, Accetta C, Di Leone A, et al. Update on oncoplastic breast surgery. Eur Rev Med Pharmacol Sci. 2012; 16:1530–1540. PMID: 23111966.
23. Schwartz T, Degnim AC, Landercasper J. Should re-excision lumpectomy rates be a quality measure in breast-conserving surgery? Ann Surg Oncol. 2013; 20:3180–3183. PMID: 23975318.

24. Landercasper J, Whitacre E, Degnim AC, Al-Hamadani M. Reasons for re-excision after lumpectomy for breast cancer: insight from the American Society of Breast Surgeons Mastery (SM) database. Ann Surg Oncol. 2014; 21:3185–3191. PMID: 25047472.
25. Savalia NB, Silverstein MJ. Oncoplastic breast reconstruction: patient selection and surgical techniques. J Surg Oncol. 2016; 113:875–882. PMID: 27004728.

26. Stankov A, Bargallo-Rocha JE, Silvio AÑ, Ramirez MT, Stankova-Ninova K, Meneses-Garcia A. Prognostic factors and recurrence in breast cancer: experience at the national cancer institute of Mexico. ISRN Oncol. 2012; 2012:825258. PMID: 22830047.

27. Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007; 9:R6. PMID: 17239243.

28. Dowsett M, Nielsen TO, A'Hern R, Bartlett J, Coombes RC, Cuzick J, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst. 2011; 103:1656–1664. PMID: 21960707.

29. Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005; 353:1784–1792. PMID: 16251534.

30. Losken A, Dugal CS, Styblo TM, Carlson GW. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg. 2014; 72:145–149. PMID: 23503430.

SUPPLEMENTARY MATERIALS
Supplementary Figure 1
Forest plot of the molecular expression states in the included cases. Weights are from random effects analysis.
RR=relative risk; CI=confidence interval; HER2=human epidermal growth factor receptor 2; ER=estrogen receptor.
Supplementary Figure 2
Forest plot of the adjuvant treatments received in the included cases. Weights are from random effects analysis.
RR=relative risk; CI=confidence interval.
Figure 2
Forest plot of the distribution of pathological stages. The pathological T (A) and N (B) stages in oncoplastic surgery (OPS) and breast-conserving surgery (BCS) groups. Weights are from random effects analysis.
RR= relative risk; CI=confidence interval.
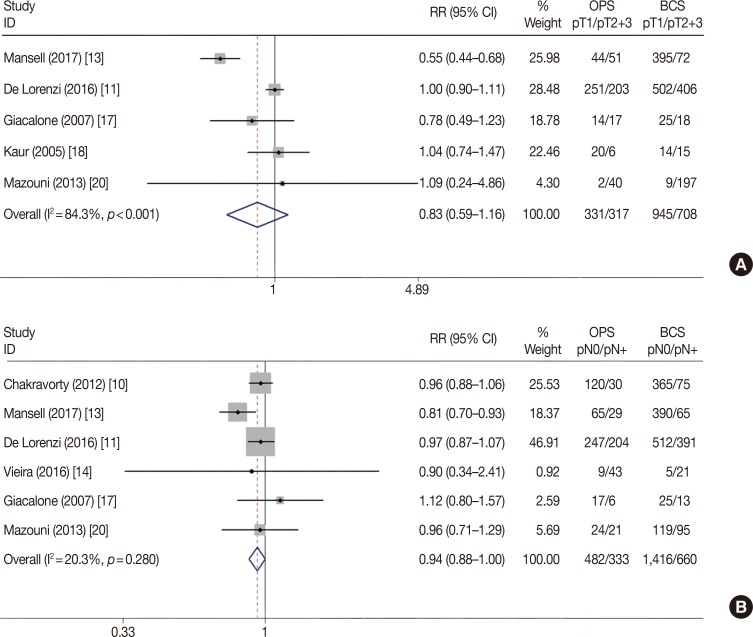
Figure 3
Forest plot of margin status difference in oncoplastic surgery (OPS) and breast-conserving surgery (BCS) groups.
RR=relative risk; CI=confidence interval.
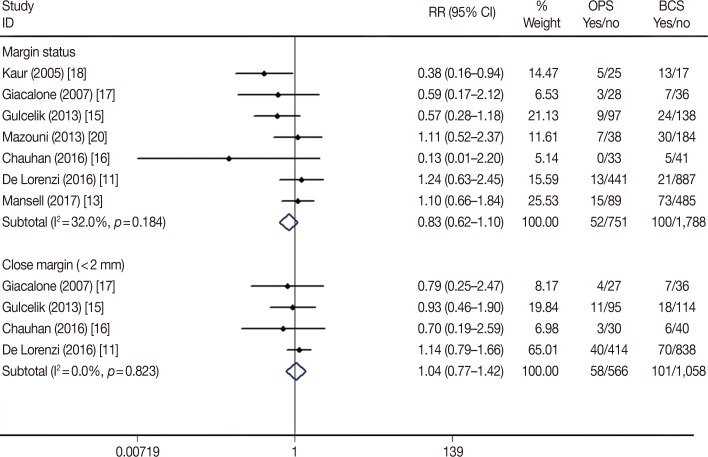
Figure 4
Forest plot of discrepancy of re-excision rate in oncoplastic surgery (OPS) and breast-conserving surgery (BCS) groups.
RR=relative risk; CI=confidence interval.
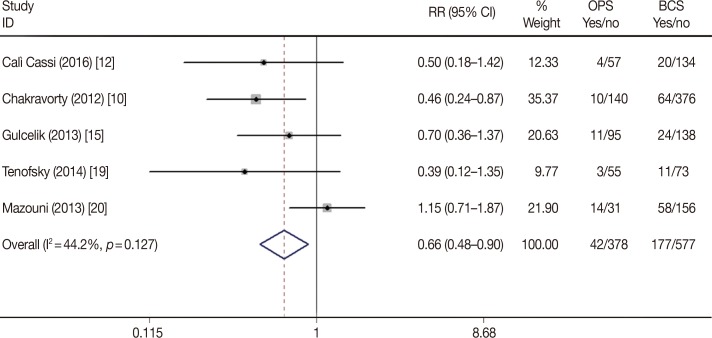
Figure 5
Forest plot of tumor recurrence differences in oncoplastic surgery (OPS) and breast-conserving surgery (BCS) groups.
RR=relative risk; CI=confidence interval.
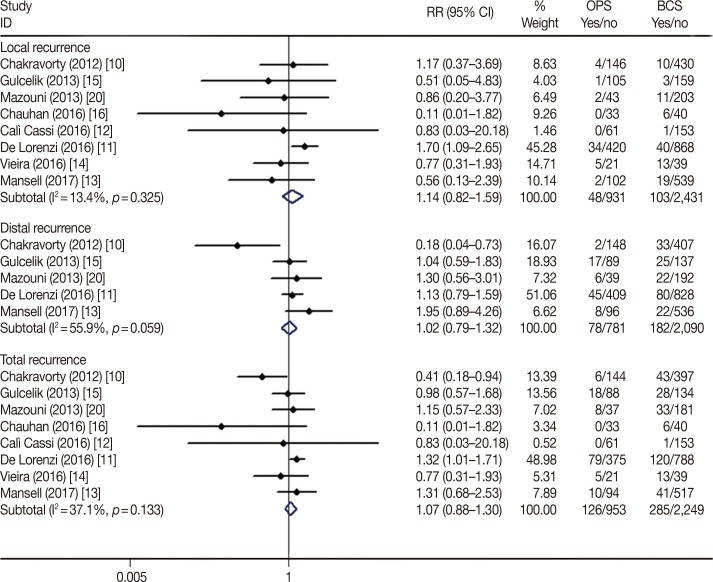
Figure 6
Forest plot of overall survival and disease-free survival difference in oncoplastic surgery (OPS) and breast-conserving surgery (BCS) groups.
HR=hazard ratio; CI=confidence interval; DFS=disease-free survival; OS=overall survival.
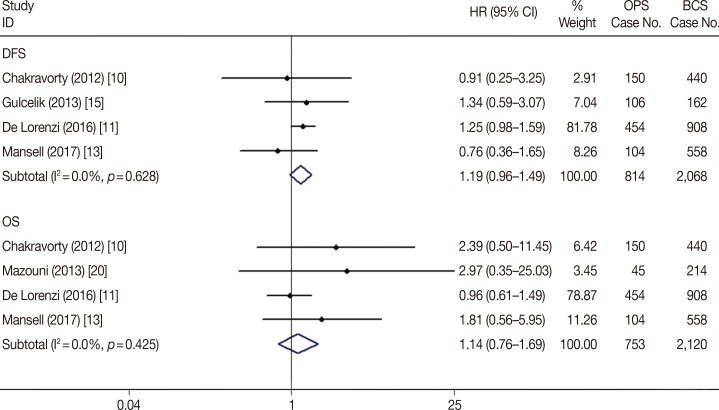
Table 1
Main characteristics of studies included in the meta-analysis
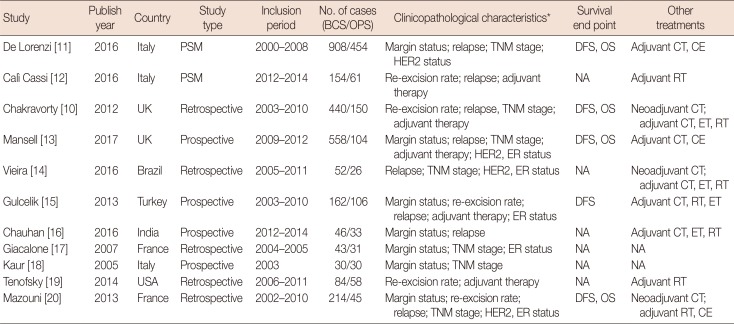
| Study | Publish year | Country | Study type | Inclusion period | No. of cases (BCS/OPS) | Clinicopathological characteristics* | Survival end point | Other treatments |
|---|---|---|---|---|---|---|---|---|
| De Lorenzi [11] | 2016 | Italy | PSM | 2000–2008 | 908/454 | Margin status; relapse; TNM stage; HER2 status | DFS, OS | Adjuvant CT, CE |
| Calì Cassi [12] | 2016 | Italy | PSM | 2012–2014 | 154/61 | Re-excision rate; relapse; adjuvant therapy | NA | Adjuvant RT |
| Chakravorty [10] | 2012 | UK | Retrospective | 2003–2010 | 440/150 | Re-excision rate; relapse, TNM stage; adjuvant therapy | DFS, OS | Neoadjuvant CT; adjuvant CT, ET, RT |
| Mansell [13] | 2017 | UK | Prospective | 2009–2012 | 558/104 | Margin status; relapse; TNM stage; adjuvant therapy; HER2, ER status | DFS, OS | Adjuvant CT, CE |
| Vieira [14] | 2016 | Brazil | Retrospective | 2005–2011 | 52/26 | Relapse; TNM stage; HER2, ER status | NA | Neoadjuvant CT; adjuvant CT, ET, RT |
| Gulcelik [15] | 2013 | Turkey | Prospective | 2003–2010 | 162/106 | Margin status; re-excision rate; relapse; adjuvant therapy; ER status | DFS | Adjuvant CT, RT, ET |
| Chauhan [16] | 2016 | India | Prospective | 2012–2014 | 46/33 | Margin status; relapse | NA | Adjuvant CT, ET, RT |
| Giacalone [17] | 2007 | France | Retrospective | 2004–2005 | 43/31 | Margin status; TNM stage; ER status | NA | NA |
| Kaur [18] | 2005 | Italy | Prospective | 2003 | 30/30 | Margin status; TNM stage | NA | NA |
| Tenofsky [19] | 2014 | USA | Retrospective | 2006–2011 | 84/58 | Re-excision rate; adjuvant therapy | NA | Adjuvant RT |
| Mazouni [20] | 2013 | France | Retrospective | 2002–2010 | 214/45 | Margin status; re-excision rate; relapse; TNM stage; HER2, ER status | DFS, OS | Neoadjuvant CT; adjuvant RT, CE |
BCS=breast-conserving surgery; OPS=oncoplastic surgery; PSM=propensity score matching; HER2=human epidermal growth factor receptor 2; DFS=disease-free survival; OS=overall survival; CT=chemotherapy; CE=chemotherapy and endocrine therapy; NA=not available; RT=radiotherapy; ET=endocrine therapy; ER=estrogen receptor.
*Only characteristics associated with prognosis included.
Table 2
Meta-analysis outcomes of the eligible studies




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download