Abstract
Purpose
Previous oncological studies showed that lymph node ratio (LNR) (ratio of number of lymph nodes that tested positive for metastasis to the total number of lymph nodes examined) is a negative indicator of cancer survival. The American Joint Committee on Cancer (AJCC) staging system incorporates tumor size, lymph node involvement, and metastasis in a comprehensive model of cancer progression, but LNR alone has been shown to outperform the AJCC system in prognostic and survival predictions for various types of cancer. The effectiveness of LNR has not been evaluated in breast cancer staging. Evaluating LNR for predicting cancer staging in breast cancer has the potential to improve treatment recommendations.
Methods
The Surveillance, Epidemiology, and End Results dataset was used to identify 10,655 breast cancer patients who underwent nodal evaluation from 2010 to 2013, and their LNRs were calculated. Descriptive statistics of lymph node evaluation in the patients are provided. Logistic regression with LNR as the continuous independent variable was conducted to determine whether LNR could predict cancer progression, coded as regional or distant. Analysis was conducted using SPSS version 24.
Results
Patient's mean age was 59.43±18.62. Logistic regression analysis revealed that for every 1.3% increase in LNR, the odds of falling into the distant stage of the TNM staging system increased by 13.7% (odds ratio, 14.73; 95% confidence interval, 12.00–18.08).
Conclusion
LNR, while correlated with breast cancer staging, serves as a better predictor of survival. Precision staging can influence treatment modality, and improved treatments can significantly improve quality of life. Additional research and diagnostic examinations using LNR as a potential tool for accurate staging in breast cancer patients are warranted.
Cancer is the second leading cause of death in the United States. A total of 1,685,210 new cancer cases were expected to be diagnosed in 2016, with 595,650 expected deaths [1]. Of these, 249,260 newly diagnosed breast cancer cases were estimated, with 40,890 expected deaths. Breast cancer makes up 14.79% of the total expected cancer diagnoses and 6.86% of the total expected cancer related deaths for both sexes in the United States. In women, breast cancer is expected to account for 29% of new cancer diagnoses. While breast cancer patients have increased survival rates in comparison with other cancer types, the high incidence places breast cancer as one of the most common causes of cancer-related deaths [1]. Since 1990, the rate of mortality from breast cancer has been declining due to improved treatments and early detection methods [2]. These have been shown to be important predictors of survival, as early detection has been associated with reduced breast cancer morbidity and mortality rates [3].
A key component in identifying the appropriate treatment course is an evaluation of the cancer at the time of diagnosis [4]. Breast cancer staging is a method of determining severity of the disease and may include a physical examination of the skin, mammary glands, and lymph nodes, with the axillary, supraclavicular, and cervical nodes as the primary nodes of evaluation [5]. Different methods exist for classifying clinical and pathological findings into stages, though the most commonly used guidelines in the world are from the American Joint Committee on Cancer (AJCC) [6]. The AJCC staging system is standardized, with specifically defined criteria for each known stage of breast cancer. The system has traditionally included the size of the tumor (T), the extent of spreading to the regional lymph nodes (N), along with the presence of metastasis to relatively distal areas (M) with numbers and lowercase letters for subtyping as needed (e.g., T1a, T1N2M0). The tumor can be measured based on its clinical features, appearance on imagery, size, and growth. Extent of spreading to regional lymph nodes is typically staged by pathology of tissue samples obtained by biopsy, which is known as pathologic staging (pN). pN staging is the most accurate way to assess nodal involvement because of the distinctive histological profiles of tumor cells. Collectively, these categories coupled with the metastasis staging are known as the TNM staging system.
Even though the extent of metastasis is critical to assess a patient's prognosis, the classifications have proven to be difficult to define further than "evidence of tumor cells in areas beyond the tumor site and regional lymph nodes." This may be due to the lack of clinical presentation of the pathological M0 stage which can only be proven at autopsy. The 7th edition of the AJCC's staging system raised this issue, yet the staging for this category remained the same in the AJCC's 8th edition. Also mentioned were isolated tumor cells which are single or small clusters of submillimeter tumor cells which could be indicative of distant metastasis. These have not been assessed for their role in cancer prognosis. With these continually unaddressed gaps in classification and additions to treatment protocols such as neoadjuvant therapy and multigene panel screening, it is important to consider the use of complementary staging systems. The AJCC released the 8th edition of its cancer staging manual in 2017 with major changes recommended for breast cancer staging [78]. The new consensus staging system maintained the TNM staging but added increased details of tumor dimension parameters, consideration of neoadjuvant therapy, and adjustments for multigene panels for cancers with known genetic etiologies that allow more flexibility and precision for breast cancer staging. Breast cancer subtypes were categorized by involvement of several hormone receptors which are commonly implicated in breast cancer: human epidermal growth factor receptor 2 (HER2), estrogen receptor (ER), and progesterone receptor (PR) [9]. Gándara-Cortes et al. [10] elucidated targeted and sensitive treatments for cancers characterized by the involvement of these receptors. Prognosis generally tended to be worse for those diagnosed with triple-negative (HER2–/ER–/PR–) and HER2-positive breast cancers, though cases within a specific subtype will vary somewhat in presentation [1112]. This combined TNM and subtype staging system is better at predicting survival for breast cancer than either staging system alone [13], indicating that even the most updated version of the AJCC manual needed the extra nuances of a supplementary staging system.
The addition of new staging criteria reflects an awareness of concerns with the TNM approach, not just in breast cancer but in multiple areas of oncology. Recently, the evaluation of lymph node ratio (LNR) in gastric cancer staging was shown to outperform the 7th edition guidelines of the AJCC's TNM staging system in sensitivity measures and overall survival [14]. LNR also proved useful in the prognosis of postsurgical pancreatic cancer patients as one of the most powerful predictors of survival time [15]. The 7th edition of the AJCC's staging system requires the examination of 15 lymph nodes for accurate staging and classifies stage by location of the involved nodes. Yet, the examination of 15 lymph nodes is often not performed as some nodes are unavailable for resection, resulting in under-staging. Increased identification of involved nodes and surgical resections of these nodes resulted in increased staging accuracy and colon cancer survival prediction [16]. Inadequate analysis of lymph node involvement is a result of certain types of operations or a tumor with few local lymph nodes for assessment. LNR has been used to augment staging determination and successfully predict prognosis [17]. Even in cases where sufficient nodal resection was performed, LNR has been shown to be superior to the AJCC's staging system via the assessment of the number of positive lymph nodes for predicting prognosis in colon cancer [18]. Survival rates predicted with LNR, metastatic lymph nodes, and log odds of positive lymph nodes as the staging method were superior to the AJCC's 7th edition TNM staging system in rare perihilar cholangiocarcinoma as well [19].
There is evidence of the prognostic value in using LNR to predict breast cancer survival from multiple small studies (sample sizes of less than 1,800) [20] and limited studies of larger size [2122]. There are a number of factors that can interfere with the value of LNR as a useful tool for prognosis in breast cancer, including very large tumor sizes or advanced disease, very-early stage disease, residual disease, or the use of neoadjuvant therapy which can interfere with nodal evaluation [23], though other research shows LNR is effective for prognostic prediction of high-risk breast cancers, even when neoadjuvant therapy is used [24]. In one study combining multiple cancer trials for an overall analysis of over 7,000 breast cancer patients, LNR was useful for prognosis in a subset of patients with 1–3 affected lymph nodes [25]. The effectiveness of LNR as a prognostic indicator has resulted in suggestions for inclusion of LNR in breast cancer staging [21].
Newer research as well as AJCC's 8th edition staging system has focused on breast cancer subtypes as an adjunct to survival prediction and will be an important part of prospective studies. However, the recent development of assays and imaging approaches to identify the genetic subtypes are still under development and range in both cost and availability [102627], making large scale analysis inaccessible at present. The purpose of this study was to access the large number of samples available in a national cancer data registry to evaluate the effectiveness of LNR in predicting breast cancer survival compared to TNM staging.
The study employed a retrospective analysis of The Surveillance, Epidemiology, and End Results (SEER) dataset. The SEER dataset collects information from 12 population-based cancer registries which comprise 14% of the United States population. Patient demographic information, cancer diagnosis, and clinical indicators such as primary tumor size, grade, and extension were obtained on 10,651 breast cancer patients who underwent nodal evaluation between 2010 and 2013. These characteristics were examined and reported with descriptive statistics (Table 1).
The definitions in the SEER summary staging manual were used to distinguish each summary stage of breast cancer. The localized stage cases were excluded from analysis because the localized stage is the only stage aside from the in situ stage that does not include any measurable or significant degree of lymph node involvement. The intrinsic nature of these stages precludes their ability to participate in the evaluation of a lymph node-based staging system. SEER defines regional lymph nodes as the most proximal lymph nodes which serve as immediate drainage sites for the site of the tumor and are therefore good indicators for the metastatic behavior of the cancer. For tumors of the breast, these include the axillary lymphatic plexus, paramammary lymph nodes, and interpectoral axillary lymph nodes.
Metastasis classifications are binary: M0 (no distant metastasis) or M1 (distant metastasis). LNR was calculated as the number of lymph nodes tested positive for metastasis after resection divided by the total number of lymph nodes that were examined. Local, regional, and distant SEER categorizations are determined by the TNM staging system and incorporate factors of tumor size, multiplicity, depth of invasion, extension to regional or remote areas, and histologic grade, in addition to lymph node involvement. Independent sample t-tests were run to test the hypothesis that staging and LNR are each associated with survival months. Correlational analysis was conducted to examine the relationship between LNR and TNM staging. Logistic regression was performed using SPSS version 24 (IBM Corp., Armonk, USA) to identify whether LNR and TNM staging could predict survival months, and whether LNR could predict cancer staging as either local, regional, or distant.
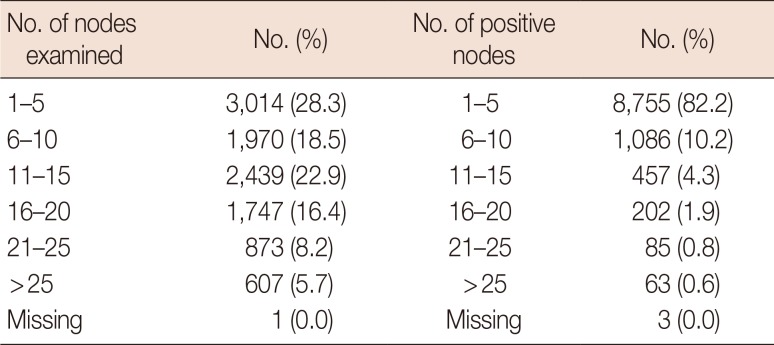
There were 10,523 women (98.8%) and 132 men (1.2%) included in the study. Patient's mean age was 59.43±18.62 (Table 1). There were 267 individuals under age 35 years; aged 35–54 years, 3,848; aged 55–75 years, 5,117; and over age 75 years, 1,423. Only two patients were in the localized group, thus these cases were excluded due to the small sample size. A total of 9,736 individuals (91.4%) were identified to be in the regional cancer stage group, and 913 (8.6%) in the distant cancer stage group (Figure 1). There were 7,423 cases (59.7%) that had 15 or fewer nodes examined (Table 2). A chi-square test was performed but no relationship was found between age and LNR (χ2(3, N=10,647)=5.03; p=0.170).
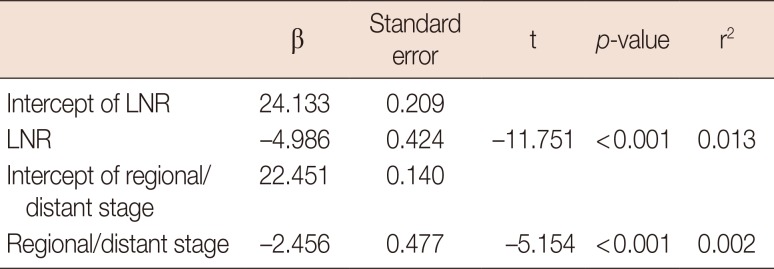
LNR and TNM staging for the classification of cases into either regional or distant cancer stages had a Spearman's rho of 0.237, which was a small but significant correlation (p<0.001). LNR was negatively correlated with survival months (r=−0.113, p<0.001) such that a lower ratio of positive nodes resulted in longer survival months. Linear regression analysis found that LNR was a stronger predictor of survival months (β=−0.113, p<0.001, r2=0.013) than TNM staging (β=−0.050, p<0.001, r2=0.002) for both regional and distant cancer stages (Table 3). Other multivariate models were tested, including the addition of age at diagnosis and breast cancer subtypes, but these variables did not alter the significance of LNR in predicting survival months (results available upon request). LNR, while more predictive of survival months, was related to TNM staging as logistic regression analysis revealed that for every 1% increase in LNR, the odds of falling into the distant cancer stage of the TNM staging system increased by 13.7% (odds ratio, 14.73; 95% confidence interval, 12.00–18.08).
In this study, we sought to evaluate the prognostic value of LNR within the breast cancer node positive cohort of the SEER dataset in comparison to the survival prediction value of the TNM staging system in SEER. It is well-established that LNR can be a strong prognosis factor for several cancer types [18]. More specifically, the prognostic use of LNR in breast cancer has been shown to be a significant predictor of patient overall survival, spanning all stages of breast cancer and various treatment types [202228]. The present analysis used a larger cohort of the SEER cancer registry than previous studies to compare the predictive value of LNR to TNM staging in breast cancer survival for a diverse patient population, which is necessary for the findings to reflect the impact on the population at large.
We found that while LNR and TNM cancer staging have a small but significant correlation, LNR was a better predictor of survival than TNM. Our results, while consistent with previous studies that showed LNR may be warranted as a superior method of breast cancer prognosis, also found that LNR exponentially increases the odds of falling into the distant stage of the TNM cancer staging system. This association between LNR and distant stage TNM potentially explains the higher prediction value of LNR over TNM staging.
The present study on a broad population of over 10,000 breast cancer patients evaluated whether LNR would be a useful prognostic indicator for today's oncology providers. LNR was able to provide a greater prediction of overall survival than TNM staging, though the r2 value suggested that just over 1% of the variation in survival months could be explained by LNR. While statistically significant, this level of prediction may not be clinically meaningful or relevant for treatment considerations for cases without node involvement, or node-negative cases. This finding was consistent with other LNR analyses that suggested only a small, defined subpopulation of breast cancer patients would benefit from the use of LNR [25]. The analysis could have benefited from the consistent implementation of active post-study follow-up. Several SEER regions input survival as a "presumed alive" date (no death certificate or autopsy of patient during the study period) as the study end date, which was December 31, 2013. Other regions proceeded with active follow-up and gave "date of last contact" (with the research staff) as date of survival.
To incorporate LNR meaningfully into clinical practice, the relationship between ratios and changes in ability to predict overall survival must be defined. The AJCC reviews their guidelines among multitudes of researchers and physicians to update the system's parameters on a regular basis. Reviews have found the range of LNR between 0.20 and 0.65 to be the most common cutoff points for increases and decreases in overall survival [29]. Future studies should investigate and suggest guidelines for evaluating these ratios.
Broad level analysis is limited by the inclusion of almost all breast cancer types and levels of disease progression, which are factors that can inhibit the usefulness of LNR in survival prediction [23]. It should be understood that the patient population chosen for analysis excluded those with in situ and localized stage diagnoses due to the nature of the lymph node biopsy procedure. Including subtype in the analysis did not alter the significance of LNR as a predictor of survival months. Additionally, the presence and extent of study variables including survival months depended on the SEER region collecting the data, as some regions provided active patient follow-up while some regions used recorded data to determine survival status. The analysis was also constrained because the SEER variable identifying sentinel lymph node biopsy versus axillary lymph node dissection was found to be prone to underestimation due to data collection procedures, making the data unavailable as a consideration in the current analysis [30]. However, the inaccurate collection procedures were rectified in 2012 and should be included in future analysis of the utility of lymph node dissection in cancer staging.
The focus in all the updates to the AJCC 8th edition guidelines is on cancers with the detectable biomarkers of ER, PR, and HER2, and cases that are post-neoadjuvant therapy. Neoadjuvant therapies are systemic treatments that are now included in therapeutic recommendations to downstage cases of advanced breast cancer prior to surgical intervention. Accurate staging of disease after the administration of neoadjuvant therapy is even more imperative due to its predictive role in determining risk of recurrence. Persistence of tumor growth in lymph nodes despite prophylactic treatment warrants a more strategic approach to treatment. For this reason, LNR is worth considering as a supplementary staging technique for the goal of attaining complete pathological response, which means there is no evidence of invasive cancer left in the patient. This is only attained when the post-neoadjuvant AJCC stage of the disease is T0/is and N0, meaning there is no primary tumor as well as no evidence of metastatic spread to the axillary lymph nodes. Of note, the use of neoadjuvant therapies will likely decrease the number of cases staged as distant metastasis, a consideration for the refinement of future LNR research to be specific and attentive to regional node metastasis. The success of LNR for the prediction of survival in the nebulous and possibly less predictable intermediate stages warrants research into a supplementary role as the impact of biologically-based intervention on prognosis changes the landscape of breast oncology. Further investigation should evaluate known breast cancer subtypes that exhibit aggressive metastasis as well as survival rates for the concurrent usage of the AJCC and LNR staging systems.
Notes
This study was supported by the Quality Outcomes Research and Assessment, Department of Orthopaedics, University of Utah School of Medicine (http://QualityOutcomesResearch.com), the Huntsman Cancer Institute, and the Center for Clinical & Translational Science, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5UL1TR001067-02.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30. PMID: 26742998.

2. Brandt J, Garne JP, Tengrup I, Manjer J. Age at diagnosis in relation to survival following breast cancer: a cohort study. World J Surg Oncol. 2015; 13:33. PMID: 25889186.

3. Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, Shih YC, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015; 314:1599–1614. PMID: 26501536.
4. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474. PMID: 20180029.

5. Greene FL. American Joint Committee on Cancer. American Cancer Society. AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag;2002.
6. Chavez-MacGregor M, Mittendorf EA, Clarke CA, Lichtensztajn DY, Hunt KK, Giordano SH. Incorporating tumor characteristics to the American Joint Committee on Cancer breast cancer staging system. Oncologist. 2017; 22:1292–1300. PMID: 28592619.

7. Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, et al. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017; 67:290–303. PMID: 28294295.

8. Amin MB. American Joint Committee on Cancer. AJCC Cancer Staging Manual. 8th ed. New York: Springer;2017. p. 589–636.
9. Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015; 107:djv048. PMID: 25825511.

10. Gándara-Cortes M, Vázquez-Boquete Á, Fernández-Rodríguez B, Viaño P, Ínsua D, Seoane-Seoane A, et al. Breast cancer subtype discrimination using standardized 4-IHC and digital image analysis. Virchows Arch. 2018; 472:195–203. PMID: 28825136.

11. Howlader N, Cronin KA, Kurian AW, Andridge R. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol Biomarkers Prev. 2018; 27:619–626. PMID: 29593010.

12. Parise CA, Caggiano V. Risk of mortality of node-negative, ER/PR/HER2 breast cancer subtypes in T1, T2, and T3 tumors. Breast Cancer Res Treat. 2017; 165:743–750. PMID: 28689363.

13. Yang ZJ, Yu Y, Chi JR, Guan M, Zhao Y, Cao XC. The combined pN stage and breast cancer subtypes in breast cancer: a better discriminator of outcome can be used to refine the 8th AJCC staging manual. Breast Cancer. 2018; 25:315–324. PMID: 29353447.

14. Lee YC, Yang PJ, Zhong Y, Clancy TE, Lin MT, Wang J. Lymph node ratio-based staging system outperforms the seventh AJCC system for gastric cancer: validation analysis with national Taiwan University Hospital Cancer Registry. Am J Clin Oncol. 2017; 40:35–41. PMID: 25089533.
15. Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007; 141:610–618. PMID: 17462460.

16. Le Voyer TE, Sigurdson ER, Hanlon AL, Mayer RJ, Macdonald JS, Catalano PJ, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003; 21:2912–2919. PMID: 12885809.

17. Kutlu OC, Watchell M, Dissanaike S. Metastatic lymph node ratio successfully predicts prognosis in Western gastric cancer patients. Surg Oncol. 2015; 24:84–88. PMID: 25912951.

18. Wang J, Hassett JM, Dayton MT, Kulaylat MN. Lymph node ratio: role in the staging of node-positive colon cancer. Ann Surg Oncol. 2008; 15:1600–1608. PMID: 18327530.

19. Conci S, Ruzzenente A, Sandri M, Bertuzzo F, Campagnaro T, Bagante F, et al. What is the most accurate lymph node staging method for perihilar cholangiocarcinoma? Comparison of UICC/AJCC pN stage, number of metastatic lymph nodes, lymph node ratio, and log odds of metastatic lymph nodes. Eur J Surg Oncol. 2017; 43:743–750. PMID: 28094085.

20. Woodward WA, Vinh-Hung V, Ueno NT, Cheng YC, Royce M, Tai P, et al. Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol. 2006; 24:2910–2916. PMID: 16782931.

21. Wu SG, Wang Y, Zhou J, Sun JY, Li FY, Lin HX, et al. Number of negative lymph nodes should be considered for incorporation into staging for breast cancer. Am J Cancer Res. 2015; 5:844–853. PMID: 25973321.
22. Vinh-Hung V, Nguyen NP, Cserni G, Truong P, Woodward W, Verkooijen HM, et al. Prognostic value of nodal ratios in node-positive breast cancer: a compiled update. Future Oncol. 2009; 5:1585–1603. PMID: 20001797.

23. Safavi A, Kaviani A, Mohammadzadeh N, Zand S, Elahi A, Krag DN. Breast cancer prognostication by pathologic node staging (pN-staging) system versus lymph node ratio (LNR): a critical review of conflicts with number of nodes, z-0011 trial, staging cut-points, neo-adjuvant therapy, and survival estimation. Arch Breast Cancer. 2017; 4:110–123.
24. Tsai J, Bertoni D, Hernandez-Boussard T, Telli ML, Wapnir IL. Lymph node ratio analysis after neoadjuvant chemotherapy is prognostic in hormone receptor-positive and triple-negative breast cancer. Ann Surg Oncol. 2016; 23:3310–3316. PMID: 27401442.

25. Tausch C, Taucher S, Dubsky P, Seifert M, Reitsamer R, Kwasny W, et al. Prognostic value of number of removed lymph nodes, number of involved lymph nodes, and lymph node ratio in 7502 breast cancer patients enrolled onto trials of the Austrian Breast and Colorectal Cancer Study Group (ABCSG). Ann Surg Oncol. 2012; 19:1808–1817. PMID: 22207051.

26. Kittaneh M, Montero AJ, Glück S. Molecular profiling for breast cancer: a comprehensive review. Biomark Cancer. 2013; 5:61–70. PMID: 24250234.

27. Kwa M, Makris A, Esteva FJ. Clinical utility of gene-expression signatures in early stage breast cancer. Nat Rev Clin Oncol. 2017; 14:595–610. PMID: 28561071.

28. Solak M, Turkoz FP, Keskin O, Aksoy S, Babacan T, Sarici F, et al. The lymph node ratio as an independent prognostic factor for non-metastatic node-positive breast cancer recurrence and mortality. J BUON. 2015; 20:737–745. PMID: 26214625.
29. Liu D, Chen Y, Deng M, Xie G, Wang J, Zhang L, et al. Lymph node ratio and breast cancer prognosis: a meta-analysis. Breast Cancer. 2014; 21:1–9. PMID: 24101545.

30. National Cancer Institute. Scope of regional lymph node surgery-SEER documentation. Accessed February 6th, 2018. https://seer.cancer.gov/seerstat/variables/seer/regional_ln/.
Figure 1
Distribution of the Surveillance, Epidemiology, and End Results population for three progressions of breast cancer stage.
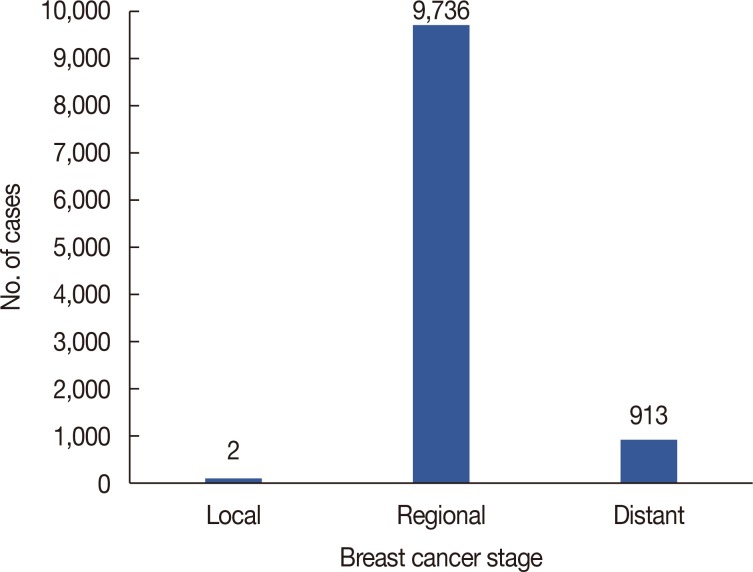
Table 1
Demographics of patients in the SEER population (n=10,655)

Table 2
Number of nodes examined and frequency of positive nodes for patients in the SEER




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download