1. Bergkvist L, de Boniface J, Jönsson PE, Ingvar C, Liljegren G, Frisell J, et al. Axillary recurrence rate after negative sentinel node biopsy in breast cancer: three-year follow-up of the Swedish Multicenter Cohort Study. Ann Surg. 2008; 247:150–156. PMID:
18156935.
2. Giuliano AE, Haigh PI, Brennan MB, Hansen NM, Kelley MC, Ye W, et al. Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer. J Clin Oncol. 2000; 18:2553–2559. PMID:
10893286.

3. Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003; 349:546–553. PMID:
12904519.

4. Ashikaga T, Krag DN, Land SR, Julian TB, Anderson SJ, Brown AM, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010; 102:111–118. PMID:
20648579.

5. Fant JS, Grant MD, Knox SM, Livingston SA, Ridl K, Jones RC, et al. Preliminary outcome analysis in patients with breast cancer and a positive sentinel lymph node who declined axillary dissection. Ann Surg Oncol. 2003; 10:126–130. PMID:
12620906.

6. Guenther JM, Hansen NM, DiFronzo LA, Giuliano AE, Collins JC, Grube BL, et al. Axillary dissection is not required for all patients with breast cancer and positive sentinel nodes. Arch Surg. 2003; 138:52–56. PMID:
12511150.

7. Jeruss JS, Winchester DJ, Sener SF, Brinkmann EM, Bilimoria MM, Barrera E Jr, et al. Axillary recurrence after sentinel node biopsy. Ann Surg Oncol. 2005; 12:34–40. PMID:
15827776.

8. Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010; 252:426–432. PMID:
20739842.
9. Giuliano AE, Ballman K, McCall L, Beitsch P, Whitworth PW, Blumencranz P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 randomized trial. Ann Surg. 2016; 264:413–420. PMID:
27513155.
10. Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017; 318:918–926. PMID:
28898379.
11. Miller CL, Specht MC, Skolny MN, Horick N, Jammallo LS, O'Toole J, et al. Risk of lymphedema after mastectomy: potential benefit of applying ACOSOG Z0011 protocol to mastectomy patients. Breast Cancer Res Treat. 2014; 144:71–77. PMID:
24500108.

12. Hong R, Dai Z, Zhu W, Xu B. Association between lymph node ratio and disease specific survival in breast cancer patients with one or two positive lymph nodes stratified by different local treatment modalities. PLoS One. 2015; 10:e0138908. PMID:
26513258.

13. Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011; 305:569–575. PMID:
21304082.
14. Le VH, Brant KN, Blackhurst DW, Schammel CM, Schammel DP, Cornett WR, et al. The impact of the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial: an institutional review. Breast. 2016; 29:117–119. PMID:
27479042.

15. Gannan E, Khoo J, Nightingale S, Suhardja TS, Lippey J, Keane H, et al. Management of early node-positive breast cancer in Australia: a multicentre study. Breast J. 2016; 22:413–419. PMID:
27095381.

16. Joyce DP, Lowery AJ, McGrath-Soo LB, Downey E, Kelly L, O'Donoghue GT, et al. Management of the axilla: has Z0011 had an impact? Ir J Med Sci. 2016; 185:145–149. PMID:
25595827.

17. Loveland-Jones CE, Ruth K, Sigurdson ER, Egleston BL, Boraas M, Bleicher RJ. Patterns of nodal staging during breast conservation surgery in the medicare patient: will the ACOSOG Z0011 trial change the pattern of care? Breast Cancer Res Treat. 2014; 143:571–577. PMID:
24442687.

18. Tsao MW, Cornacchi SD, Hodgson N, Simunovic M, Thabane L, Cheng J, et al. A population-based study of the effects of a regional guideline for completion axillary lymph node dissection on axillary surgery in patients with breast cancer. Ann Surg Oncol. 2016; 23:3354–3364. PMID:
27342830.

19. Verheuvel NC, Voogd AC, Tjan-Heijnen VC, Roumen RM. Potential impact of application of Z0011 derived criteria to omit axillary lymph node dissection in node positive breast cancer patients. Eur J Surg Oncol. 2016; 42:1162–1168. PMID:
27265036.

20. Bonneau C, Hequet D, Estevez JP, Pouget N, Rouzier R. Impact of axillary dissection in women with invasive breast cancer who do not fit the Z0011 ACOSOG trial because of three or more metastatic sentinel lymph nodes. Eur J Surg Oncol. 2015; 41:998–1004. PMID:
25986854.

21. Clinical practice guidelines in oncology: breast, version 2012. National Comprehensive Cancer Network. 2012. Accessed March 26th.
http://www.nccn.org.
22. Roberts A, Nofech-Mozes S, Youngson B, McCready DR, Al-Assi M, Ramkumar S, et al. The importance of applying ACOSOG Z0011 criteria in the axillary management of invasive lobular carcinoma: a multiinstitutional cohort study. Ann Surg Oncol. 2015; 22:3397–3401. PMID:
26215196.

23. Edge SB. American Joint Committee on Cancer. American Cancer Society. American College of Surgeons. AJCC Cancer Staging Manual. 7th ed. New York: Springer;2010.
24. Caudle AS, Hunt KK, Tucker SL, Hoffman K, Gainer SM, Lucci A, et al. American College of Surgeons Oncology Group (ACOSOG) Z0011: impact on surgeon practice patterns. Ann Surg Oncol. 2012; 19:3144–3151. PMID:
22847123.

25. Delpech Y, Bricou A, Lousquy R, Hudry D, Jankowski C, Willecocq C, et al. The exportability of the ACOSOG Z0011 criteria for omitting axillary lymph node dissection after positive sentinel lymph node biopsy findings: a multicenter study. Ann Surg Oncol. 2013; 20:2556–2561. PMID:
23456432.

26. Subhedar P, Stempel M, Eaton A, Morrow M, Gemignani ML. Do the ACOSOG Z0011 criteria affect the number of sentinel lymph nodes removed? Ann Surg Oncol. 2015; 22(Suppl 3):S470–S475. PMID:
26178759.



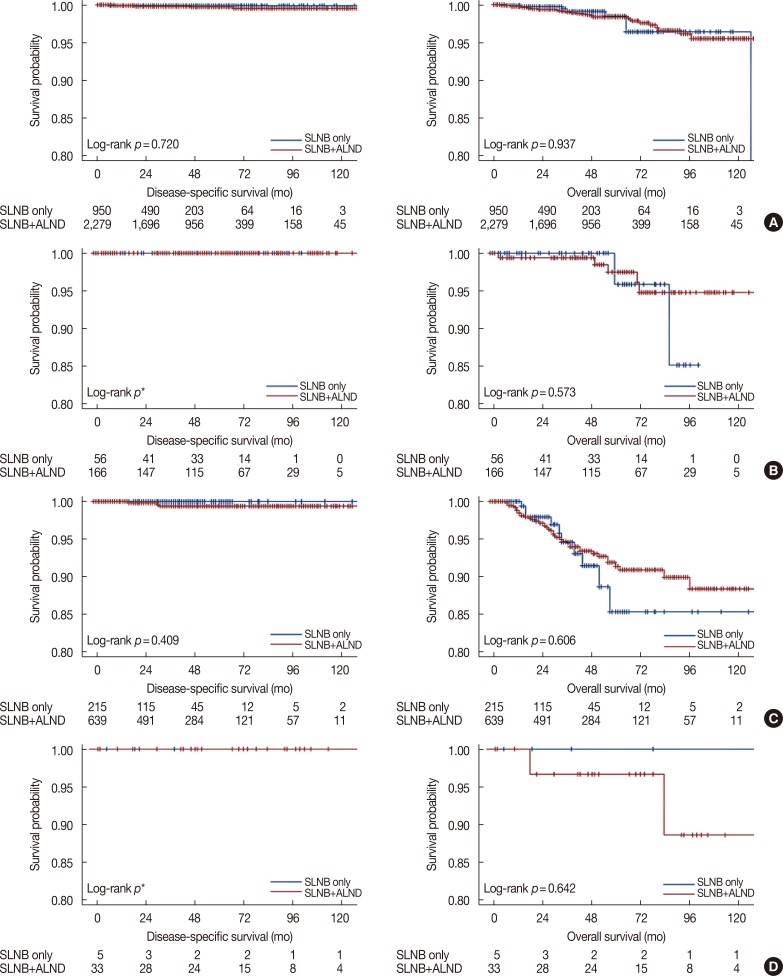
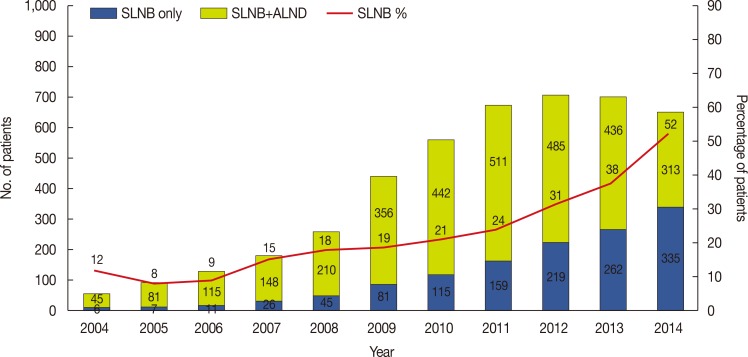
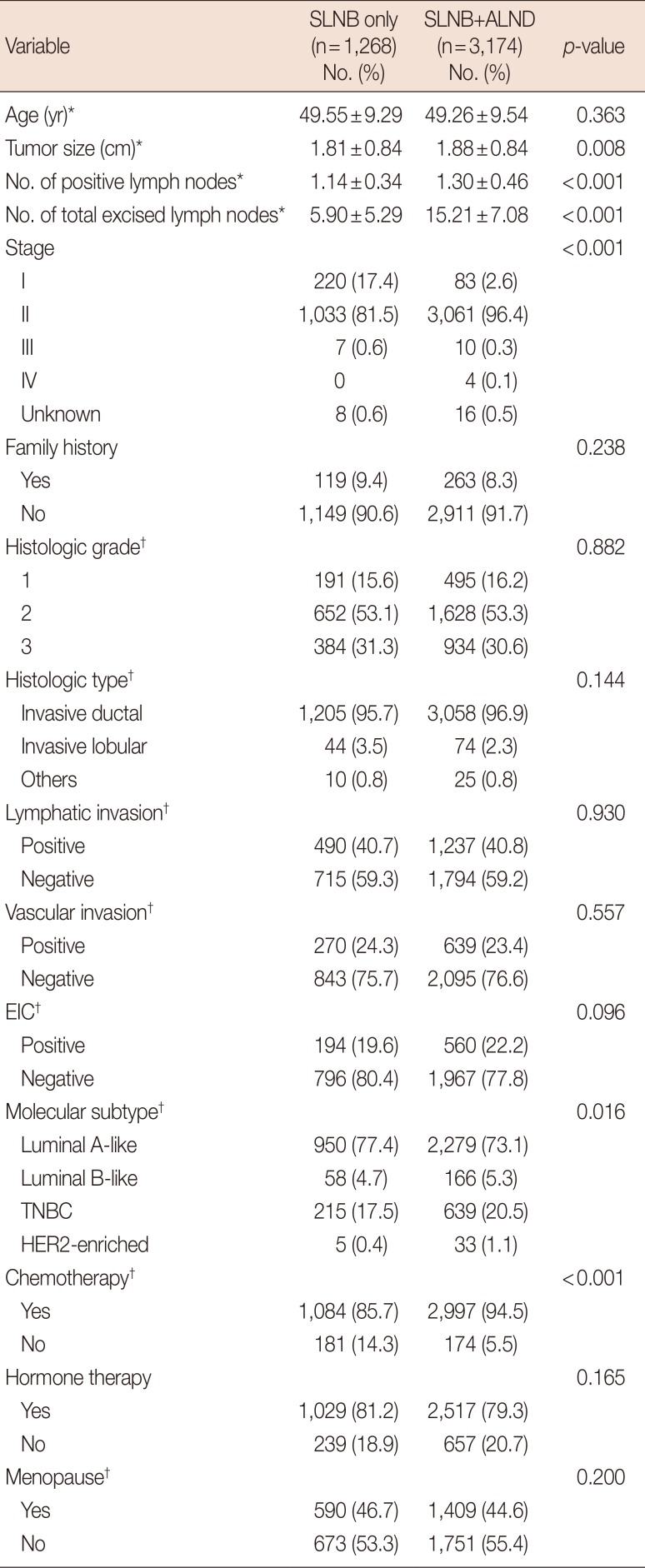
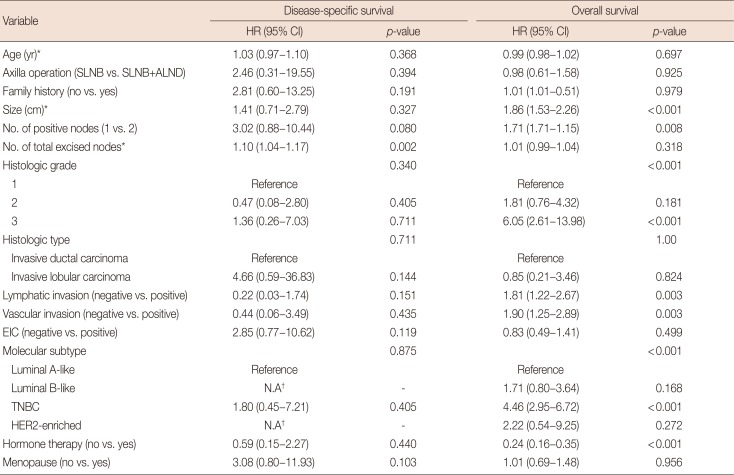




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download