Abstract
Robotic surgical systems enhance surgical accuracy and efficiency by applying advanced technologies such as artificial arm joints to provide higher degrees of freedom of movement and high-quality three-dimensional images. However, the application of robotic surgical systems to breast surgery has not been widely attempted. The robotic system would improve cosmesis by enabling surgery using a single small incision. We report the first case of a gasless robot-assisted nipple-sparing mastectomy and immediate reconstruction in a patient with early breast cancer.
A nipple-sparing mastectomy is performed to improve a patient's quality of life without compromising oncological safety in patients with early breast cancer [1]. Nipple-sparing mastectomies are rapidly gaining popularity [2], and various techniques have been introduced for this operation to achieve better outcomes not only in terms of oncological safety but also in terms of aesthetic improvement [3].
Robotic surgical systems have been applied in various surgeries for solid tumors including gastric, thyroid, colorectal, prostate, and liver tumors. Robotic surgery demonstrates unique technical features compared with endoscopic or laparoscopic surgery. Robotic surgical systems enhance surgical accuracy and efficiency by applying advanced technologies such as artificial arm joints to provide higher degrees of freedom of movement and high-quality three-dimensional images [4]. However, robotic surgical systems have not been widely applied for breast surgery.
With recent advances in robotic technologies, the use of robotic surgical systems for breast surgery could potentially improve patient satisfaction and ensure oncological safety [5]. Previous reports have suggested that the initial application of robotic surgical systems for oncological surgery in patients with breast cancer or prophylactic surgery for high-risk patients is feasible and safe [56]. We report the first case of a gasless robot-assisted nipple-sparing mastectomy with immediate reconstruction in a patient with early breast cancer.
A 49-year-old woman with a history of transsphenoidal pituitary adenoma surgery visited the outpatient clinic owing to microcalcification detected on a screening mammogram in 2016. Physical examination showed nonspecific findings with no palpable lesions. Breast ultrasonography showed a probably benign hypoechoic lesion measuring 7 mm at the 2 o'clock position in her right breast. Magnification mammography showed several grouped microcalcifications with amorphous features in the upper outer and inner quadrants of the right breast, and they classified as a Breast Imaging Reporting and Data System category 4a lesion. She had undergone a stereotactic biopsy using an 11-gauge needle with the vacuum-assisted breast biopsy system. The biopsy revealed low-grade ductal carcinoma in situ. Additional magnetic resonance imaging showed multicentric suspicious masses with suspicious enhancement in the upper, central, and medial areas of the right breast. Suspicious non-mass enhancement was observed in the upper inner to the central areas of the left breast, suggesting malignant involvement. Other preoperative evaluation including chest X-ray, electrocardiogram, and laboratory tests showed no significant abnormalities.
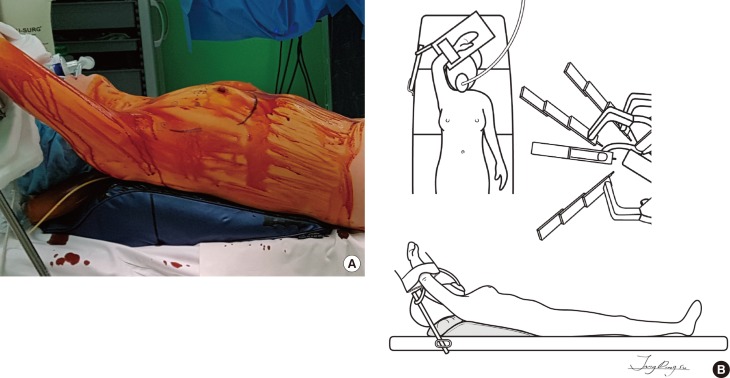
Under general anesthesia, the patient was placed in a supine position with a shoulder pad under the ipsilateral chest wall. The ipsilateral arm was straightened to the head and fixed to an arm board (Figure 1).
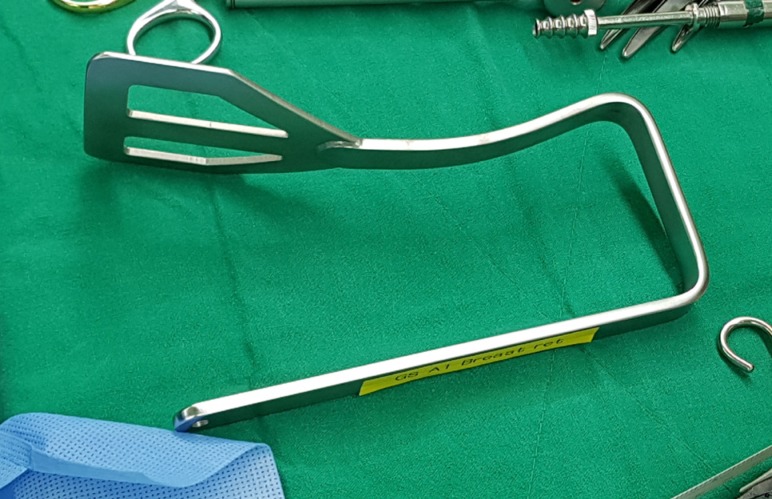
A 6-cm vertical skin incision was made in the anterior axillary line, and a sentinel lymph node biopsy was performed. Radioisotopes and the indigo carmine dye were used for the detection of sentinel lymph nodes. After the sentinel lymph node biopsy, a subcutaneous skin flap was prepared through that incision toward the nipple-areolar complex using electrocautery under direct vision. An intraoperative frozen section was performed to confirm a negative tumor margin. After undermining a subcutaneous skin flap to the nipple-areolar complex from the axilla, the Modified Chung's retractor (external retractor) (Figure 2) [7] was placed under the skin flap with a table mount lift to maintain the working space.
A dual-channel 30-degree down telescope was placed on the central arm. Fenestrated bipolar forceps and a permanent cautery spatula were placed on both sides of the scope. During the procedure, ProGrasp forceps were placed on the left side of the spatula to retract and counter-retract the breast parenchyma. The da Vinci Xi Surgical System® (Intuitive Surgical, Sunnyvale, USA) was used for this surgery (Figure 3).
Breast boundaries were marked using 0.5 mL of indigo carmine injections every 2 cm before robotic dissection. Using the robotic arms, superficial subcutaneous tissue was dissected below the nipple-areolar complex to the breast borders, including the outer, inner, upper, and lower margins. The first assistant checked the state of the flap by observing the degree of illumination (visual inspection). The deep layer was subsequently dissected from the lateral margin of the pectoral muscle fascia to the entire deep layer of the retromammary tissues. Full mobilization of the breast parenchyma was completed, and the specimen was removed through the axillary incision.
After specimen removal, manual inspection of the subcutaneous flap was performed. Manual inspection revealed that the skin flap in the upper area was slightly thicker than 5 mm. Additional subcutaneous flap shaving was performed using Metzenbaum scissors. Using the robotic arms, the plastic surgeons inserted the tissue expander through the axillary incision. Two drain tubes were inserted—one inside and the other outside the pectoralis pocket.
The total operation time was 409 minutes. The console time was 132 minutes for mastectomy and 25 minutes for reconstruction. The remaining time included waiting for the sentinel node biopsy results, constructing the skin flap, docking the robots, awaiting the arrival of the plastic surgery team, and skin incision closure. Intraoperatively, her urine output was 2,280 mL and mild hypotension was observed, which were suspicious for central diabetes insipidus. Desmopressin was administrated in the recovery room, and she was referred to the intensive care unit for early postoperative care. However, her postoperative electrolyte levels and osmolality tests showed no evidence of central diabetes insipidus, and she could be transferred to the general ward on postoperative day 1. She was discharged without any other postoperative complications on postoperative day 9.
Her final histopathological examination revealed two foci of invasive ductal carcinoma measuring 1.1 cm and 0.8 cm with extensive intraductal components. The maximum dia-meter of the invasive and in situ carcinoma was 3.2 cm. No axillary lymph node metastasis was identified. Immunohistochemical evaluation showed positive estrogen receptor and negative human epidermal growth factor receptor 2 expression. The Ki-67 index, which was calculated using the Roche iScan (Ventana, Tucson, USA) and the Ki-67 (30-9) antibody was 13.7%. The superficial margin of the primary specimen showed small foci (<1–2 mm) of abutting carcinoma, and tissues of additional flap shavings after removal of the primary breast specimen were not available to examine the final histopathological features. Thus, postoperative radiation was recommended by the multidisciplinary team at our hospital. Although she refused to undergo the OncotypeDx™ (Genomic Health, Redwood City, USA) test, systemic chemotherapy was not recommended by the multidisciplinary team. Endocrine therapy using tamoxifen was initiated 3 weeks postoperatively. Intensity-modulated radiation therapy was performed for 14 days, and the total dose of radiation administered was 3,400 cGy. Follow-up studies including breast ultrasonography and mammography performed for 1-year postoperative routine surveillance showed no recurrence.
To our knowledge, this is the first case of a gasless robot-assisted nipple-sparing mastectomy with immediate reconstruction in a patient with early breast cancer. Toesca et al. [8] first described robot-assisted nipple-sparing mastectomy with immediate reconstruction using a single-port device with carbon dioxide (CO2) gas insufflation in women with breast cancer or BRCA mutations. Sarfati et al. [6] reported robot-assisted nipple-sparing mastectomy using CO2 gas insufflation and trocars in a woman with BRCA mutation. In this case, we used a retractor during robotic surgery without CO2 gas insufflation. The advantage of the gasless technique is that it prevents postoperative complications associated with gas insufflation including subcutaneous emphysema and hypercarbia following CO2 insufflation [9]. However, ischemic changes in the skin secondary to retraction can be considered a potential disadvantage of this technique, although no ischemic changes in skin were observed in this case. Further analyses are needed to evaluate the implications of the two different techniques in robot-assisted nipple-sparing mastectomy.
A hidden axillary scar is associated with better cosmesis and patient satisfaction and serves as an advantage of robot-assisted nipple-sparing mastectomy. An axillary scar is inevitable in most patients with breast cancer because axillary staging procedures including a sentinel lymph node biopsy or axillary node dissection are essential standard procedures for breast cancer surgery. Therefore, although an axillary incision is inevitable, one that can be hidden is a major advantage of this procedure. Endoscopic skin-sparing mastectomy is at a disadvantage when approaching the lateral border of the sternum secondary to obstruction of the visual field by the mounting breast parenchyma. In selected patients, the scar can be skillfully hidden using an inframammary skin fold incision with a concomitant axillary incision. The better ergonomic features of robotic surgical systems ensure that a nipple-sparing mastectomy is easier to perform than a conventional nipple-sparing mastectomy through a small inframammary fold incision. This advantage could lead to wider application of nipple-sparing mastectomy with a hidden scar.
In this case, no recurrence was observed a year postoperatively, even though an abutting tumor was identified in the superficial margin of the resected specimen. Positive margin involvement was observed in 10% of mastectomies and 38% of skin-sparing mastectomies [1011]. Although a positive superficial margin is associated with greater rates of local recurrence, the overall rate of local recurrence is low, and no differences are observed in overall survival [1213]. In this case, postmastectomy radiation therapy (PMRT) was performed to reduce the possibility of recurrence. Previous studies have suggested that patients with a positive superficial margin and additional PMRT showed higher disease-free survival rates than those observed in patients without additional PMRT [1214].
In conclusion, gasless robot-assisted nipple-sparing mastectomy can be feasible and safe because the essential principles used for robot-assisted nipple-sparing mastectomy are similar to those for conventional nipple-sparing mastectomy. The use of advanced robotic surgical systems for breast surgery may serve as an additional treatment strategy in selected patients with breast cancer or high-risk women with genetic susceptibility.
ACKNOWLEDGMENTS
The authors would like to thank Dong-Su Jang, MFA, (Medical Illustrator) for his help with the illustrations.
Notes
References
1. Tokin C, Weiss A, Wang-Rodriguez J, Blair SL. Oncologic safety of skin-sparing and nipple-sparing mastectomy: a discussion and review of the literature. Int J Surg Oncol. 2012; 2012:921821. PMID: 22848803.

2. Laronga C, Smith P. Nipple-sparing mastectomy: an oncologic and cosmetic perspective. Surg Oncol Clin N Am. 2014; 23:549–566. PMID: 24882350.
3. Munhoz AM, Montag E, Filassi JR, Gemperli R. Immediate nipple-areola-sparing mastectomy reconstruction: an update on oncological and reconstruction techniques. World J Clin Oncol. 2014; 5:478–494. PMID: 25114861.

4. Peters BS, Armijo PR, Krause C, Choudhury SA, Oleynikov D. Review of emerging surgical robotic technology. Surg Endosc. 2018; 32:1636–1655. PMID: 29442240.

5. Toesca A, Peradze N, Manconi A, Galimberti V, Intra M, Colleoni M, et al. Robotic nipple-sparing mastectomy for the treatment of breast cancer: feasibility and safety study. Breast. 2017; 31:51–56. PMID: 27810700.

6. Sarfati B, Honart JF, Leymarie N, Rimareix F, Al Khashnam H, Kolb F. Robotic da Vinci Xi-assisted nipple-sparing mastectomy: first clinical report. Breast J. 2018; 24:373–376. PMID: 29251382.

7. Lee J, Chung WY. Robotic surgery for thyroid disease. Eur Thyroid J. 2013; 2:93–101. PMID: 24783046.

8. Toesca A, Peradze N, Galimberti V, Manconi A, Intra M, Gentilini O, et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with implant: first report of surgical technique. Ann Surg. 2017; 266:e28–e30.
9. Gottlieb A, Sprung J, Zheng XM, Gagner M. Massive subcutaneous emphysema and severe hypercarbia in a patient during endoscopic transcervical parathyroidectomy using carbon dioxide insufflation. Anesth Analg. 1997; 84:1154–1156. PMID: 9141952.

10. Yu J, Al Mushawah F, Taylor ME, Cyr AE, Gillanders WE, Aft RL, et al. Compromised margins following mastectomy for stage I-III invasive breast cancer. J Surg Res. 2012; 177:102–108. PMID: 22520579.

11. Cao D, Tsangaris TN, Kouprina N, Wu LS, Balch CM, Vang R, et al. The superficial margin of the skin-sparing mastectomy for breast carcinoma: factors predicting involvement and efficacy of additional margin sampling. Ann Surg Oncol. 2008; 15:1330–1340. PMID: 18246402.

12. Childs SK, Chen YH, Duggan MM, Golshan M, Pochebit S, Wong JS, et al. Surgical margins and the risk of local-regional recurrence after mastectomy without radiation therapy. Int J Radiat Oncol Biol Phys. 2012; 84:1133–1138. PMID: 22543200.

13. Bryan ML, D'Agostino RB, Brown DR, Howard-McNatt MM. Is postmastectomy radiation therapy indicated in patients with close or positive margins? Adv Breast Cancer Res. 2016; 5:66–73.

14. Childs SK, Chen YH, Duggan MM, Golshan M, Pochebit S, Punglia RS, et al. Impact of margin status on local recurrence after mastectomy for ductal carcinoma in situ. Int J Radiat Oncol Biol Phys. 2013; 85:948–952. PMID: 22975615.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download