Abstract
In this study, we used next-generation sequencing methods to screen 300 individuals for BRCA1 and BRCA2. A novel mutation (c.849dupT) in BRCA2 was identified in a female patient and her unaffected brothers. This mutation leads to the truncation of BRCA2 functional domains. Moreover, BRCA2 mRNA expression levels in mutation carriers are significantly reduced compared to noncarriers. Immunofluorescence and western blot assays showed that this mutation resulted in reduced BRCA2 protein expression. Thus, we identified a novel mutation that damaged the function and expression of BRCA2 in a family with breast cancer history. The pedigree analysis suggested that this mutation is strongly associated with familial breast cancer. Genetic counsellors suggest that mutation carriers in this family undergo routine screening for breast cancer, as well as other malignancies, such as prostate and ovarian cancer. The effects of this BRCA2 mutation on drug resistance should be taken into consideration during treatment.
Breast cancer has become one of the most common female malignancies worldwide, accounting for 25% of cancer diagnoses among women [1]. Although male breast cancer is rare, accounting for approximately 1% of all breast cancers, the incidence of male breast cancer is also rising by 1.1% per year [2]. The etiology of breast cancer is still largely unknown. It is widely accepted that clinical disorders, obesity, liver disease, and genetic factors represent risk factors for breast cancer. A large number of genetic studies on breast cancer have supported early prevention and personalized treatment.
Mutations in several genes, including BRCA1, BRCA2, TP53, CHEK2, PALB2, and BRIP1, are reported to increase the risk of breast cancer [345678]. Importantly, mutations in BRCA1 and BRCA2 contribute to approximately 5% of breast cancer cases [9]. Moreover, up to 25% of familial breast cancer cases are reported to result from pathogenic mutations in BRCA1 and BRCA2 [10]. Deleterious mutations in BRCA1 or BRCA2 not only lead to breast cancer, but also increase the risk of secondary malignancies, such as prostate cancer, pancreatic cancer, and gastrointestinal cancer [11]. Therefore, genetic testing of BRCA1 and BRCA2 has proven to be a powerful tool to support early prevention and appropriate personalized treatment for breast cancer in the clinic.
In this study, genetics screening of BRCA1 and BRCA2 was performed in patients with breast cancer. All genetic studies were approved by the ethics committee of the Wuhan Red Cross Hospital (approve number: WHSYYY2017003). All patients provided written informed consent in accordance to the Declaration of Helsinki.
Through our screening program, pathogenic mutations were identified in patients with breast cancer using next-generation sequencing methods. However, most of these mutations have been previously reported. Interestingly, a novel heterozygous mutation in BRCA2 (c.849dupT, p.G284Wfs*11) was detected in one family with a history of breast cancer (Figure 1A). This mutation was then confirmed by Sanger sequencing, as shown in Figure 1B. In this family, the female proband (III:6) and her two brothers (III:3, III:7) were carriers, while other members did not carry the mutation (II:1, III:2, IV:3). These results suggest that the BRCA2 mutation in these three members (III:3, III:6, III:7) was likely inherited from their mother (II:2), who was diagnosed with and died from breast cancer.
BRCA2 is localized at 13q13.1 and the length of BRCA2 is 3,418 amino acids. Key functional domains of the BRCA2 protein include the Breast Cancer (BRC) repeats, a DNA binding domain, and the C-terminal testicular receptor 2 (TR2) domain. These domains are critical for the interaction of BRCA2 with DNA or binding partners such as PALB2 and RAD51 [1213]. Moreover, distinct nuclear localization signals (NLSs) in the C-terminal region are essential for its nuclear localization and functions. BRCA2 is essential for DNA repair, DNA replication, telomere homeostasis and cell cycle progression [141516]. The mutation in BRCA2 was located on exon 7. This mutation introduces a stop codon after the 295th amino acid, leading to premature termination of translation. As shown in Figure 1C, the mutant BRCA2 protein lacks nearly all functional domains, including the BRC repeats, the α-helical domain, the oligonucleotide binding domain, and the NLS, and retains only the N-terminal region of BRCA2.
Truncations of large fragments always result in downregulation of genes at the mRNA and protein levels via nonsense mutation-mediated mRNA decay. The whole blood samples of the affected female, her younger brother and three noncarrier controls were collected for BRCA2 mRNA expression testing. As shown in Figure 1D, BRCA2 mRNA expression levels in the blood of the two mutation carriers were reduced by approximately 70% and 90% relative to noncarriers. Furthermore, the mutation-associated downregulation of BRCA2 protein was confirmed by immunofluorescence and western blot assays. As shown in Figure 2A, after overexpression in HEK293T cells, wild-type BRCA2 was expressed in both the nucleus and cytoplasm of cells, as detected by Flag antibody. However, mutant BRCA2 protein could not be detected in the nucleus or cytoplasm. In addition, as shown in Figure 2B, wild-type BRCA2 could be detected with an anti-Flag antibody using western blot. However, mutant BRCA2 protein could not be detected. In summary, these in vivo and in vitro assay results suggested that the mutation (c.849dupT, p. G284Wfs*11) could lead to a significant decrease of BRCA2 expression and therefore be damaging to the function of BRCA2.
By carrying the mutation in BRCA2, the female proband is at a greater risk for right breast cancer and other malignancies, such as ovarian cancer. Therefore, routine examinations of the right breast and other organs are indispensable. Moreover, since cells with BRCA1/2 mutations are more sensitive to DNA cross-linking drugs, such as cisplatin and carboplatin, and less susceptible to taxanes [1718], the effects of this mutation on drug sensitivity should be taken into consideration during treatment. Considering the positive family history and pathogenicity of this mutation, gene testing for other family members was also necessary and meaningful. Another two males were also identified as carriers of the mutation. Although the proband's two brothers had not been diagnosed with any malignancies yet, greater attention should be paid to breast and examinations for other cancers, such as prostate, colon and genitourinary cancer, as 4% to 14% of males with BRCA2 mutations will develop breast cancer at any age [1920], and men under 65 years old with BRCA2 mutations have a 8.6-fold higher risk of developing prostate cancer [21]. Moreover, this mutation was likely to be inherited by the brothers' offspring, who have not yet undergone genetic testing. Therefore, we recommended that these individuals undergo gene screening as soon as possible.
In this study, we identified a novel heterozygous mutation of BRCA2 in a family with a history of breast cancer. Pedigree analysis suggested that the novel mutation could be a highly pathogenic factor in familial breast cancer. In vivo and in vitro studies demonstrated that this frameshift mutation led to BRCA2 mRNA decay and that it could be damaging for BRCA2 function. Our finding not only enriches the Breast Cancer Information Core database (BICD; http://research.nhgri.nih.gov/bic/), but also provides assistance to genetic counseling for this family. More importantly, genetic counseling based on BRCA1/BRCA2 testing was clinically beneficial and significant for this family, as the results contributed to personalized treatments for the patients and prevention and early detection of breast cancer or other malignancies in the family members.
Figures and Tables
 | Figure 1A novel mutation in BRCA2. (A) Pedigree of the family with BRCA2 mutation. The family member II:1, III:2, III:3, III:6, III:7, and IV:3 were screened for the BRCA1 and BRCA2 gene, and III:3, III:6, III:7 carry the novel mutation. (B) The mutation (c.849dupT, p.G284Wfs*11) in BRCA2 identified in this family was confirmed by Sanger sequencing. (C) The diagram of human BRCA2 protein with the location of its variant identified. The PALB2 binding domain in the N-terminal is represented by green box. The blue boxes represent the Breast Cancer (BRC) repeats, which form the RAD51 binding domain. The α-helix domain (HD) is indicated by a red box. The gray boxes denote the oligonucleotide binding (OB) domain. HD and OB domains are important for single strand DNA (ssDNA) binding. The yellow boxes indicate the nuclear localization signal (NLS). NLS is also essential for RAD51 binding. The mutation (G284Wfs*11) contributed to the truncation of BRC repeats, HD domain, OB domain and NLS. (D) The real-time quantitative polymerase chain reaction demonstrated that BRCA2 mRNA expression in female and male carriers was significantly lower than that in noncarriers.LC=lung cancer; BC=breast cancer; WT=wild-type; Mut=mutant; PALB2=partner and localizer of BRCA2; GAPDH=glyceraldehyde-3-phosphate dehydrogenase.
|
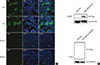 | Figure 2The abolished expression of the mutant BRCA2 in vitro. (A) The immunofluorescent demonstrated that wild-type (WT) of BRCA2 was overexpressed in both nucleus and cytoplasm of HEK293T cells which could be detected by anti-Flag antibody. However, mutant BRCA2 protein could not be detected in HEK293T cells, which were transiently transfected with the Flag-tagged mutant BRCA2 plasmids. (B) Western blotting also showed that wild-type of BRCA2 was successfully detected by anti-Flag antibody, and that mutant BRCA2 protein could not be detected in HEK293T cells.DAPI=4´,6-diamidino-2-phenylindole; NC=negative control; GAPDH=glyceraldehyde-3-phosphate dehydrogenase.
|
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–E386.

2. Ly D, Forman D, Ferlay J, Brinton LA, Cook MB. An international comparison of male and female breast cancer incidence rates. Int J Cancer. 2013; 132:1918–1926.

3. Patel UA, Perry M, Crane-Robinson C. Screening for germline mutations of the p53 gene in familial breast cancer patients. Eur J Clin Invest. 1995; 25:132–137.

4. Apostolou P, Papasotiriou I. Current perspectives on CHEK2 mutations in breast cancer. Breast Cancer (Dove Med Press). 2017; 9:331–335.

5. Cao WM, Gao Y, Yang HJ, Xie SN, Ding XW, Pan ZW, et al. Novel germline mutations and unclassified variants of BRCA1 and BRCA2 genes in Chinese women with familial breast/ovarian cancer. BMC Cancer. 2016; 16:64.

6. Ma J, Yang J, Jian W, Wang X, Xiao D, Xia W, et al. A novel loss-of-function heterozygous BRCA2 c.8946_8947delAG mutation found in a Chinese woman with family history of breast cancer. J Cancer Res Clin Oncol. 2017; 143:631–637.

7. Hofstatter EW, Domchek SM, Miron A, Garber J, Wang M, Componeschi K, et al. PALB2 mutations in familial breast and pancreatic cancer. Fam Cancer. 2011; 10:225–231.

8. Ouhtit A, Gupta I, Shaikh Z. BRIP1, a potential candidate gene in development of non-BRCA1/2 breast cancer. Front Biosci (Elite Ed). 2016; 8:289–298.

9. Antoniou AC, Easton DF. Models of genetic susceptibility to breast cancer. Oncogene. 2006; 25:5898–5905.

10. Skol AD, Sasaki MM, Onel K. The genetics of breast cancer risk in the post-genome era: thoughts on study design to move past BRCA and towards clinical relevance. Breast Cancer Res. 2016; 18:99.

11. Bayraktar S, Arun B. BRCA mutation genetic testing implications in the United States. Breast. 2017; 31:224–232.

12. Yang H, Jeffrey PD, Miller J, Kinnucan E, Sun Y, Thoma NH, et al. BRCA2 function in DNA binding and recombination from a BRCA2-DSS1-ssDNA structure. Science. 2002; 297:1837–1848.

13. Bork P, Blomberg N, Nilges M. Internal repeats in the BRCA2 protein sequence. Nat Genet. 1996; 13:22–23.

14. Helleday T, Eshtad S, Nik-Zainal S. Mechanisms underlying mutational signatures in human cancers. Nat Rev Genet. 2014; 15:585–598.

15. Lomonosov M, Anand S, Sangrithi M, Davies R, Venkitaraman AR. Stabilization of stalled DNA replication forks by the BRCA2 breast cancer susceptibility protein. Genes Dev. 2003; 17:3017–3022.

16. Min J, Choi ES, Hwang K, Kim J, Sampath S, Venkitaraman AR, et al. The breast cancer susceptibility gene BRCA2 is required for the maintenance of telomere homeostasis. J Biol Chem. 2012; 287:5091–5101.

17. Nientiedt C, Heller M, Endris V, Volckmar AL, Zschäbitz S, Tapia-Laliena MA, et al. Mutations in BRCA2 and taxane resistance in prostate cancer. Sci Rep. 2017; 7:4574.

18. Dhillon KK, Swisher EM, Taniguchi T. Secondary mutations of BRCA1/2 and drug resistance. Cancer Sci. 2011; 102:663–669.

19. Basham VM, Lipscombe JM, Ward JM, Gayther SA, Ponder BA, Easton DF, et al. BRCA1 and BRCA2 mutations in a population-based study of male breast cancer. Breast Cancer Res. 2002; 4:R2.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download