Abstract
Purpose
The Glycyrrhiza uralensis species (Leguminosae) as a medicinal biocompound, and one of its root components, isoliquritigenin (ISL), which is a flavonoid, has been reported to have anti-tumor activity in vitro and in vivo. However, its function in bone formation has not been studied yet. In this study, we tested the effect of Glycyrrhiza uralensis (ErLR) and baked Glycyrrhiza uralensis (EdLR) extracts on osteoblast proliferation, alkaline phosphatase (ALP) activity, and bone-related gene expression in osteoblastic MC3T3-E1 cells. Methods: MC3T3-E1 cells were cultured in various levels of ErLR (0, 5, 10, 15, 20 μg/mL), EdLR (0, 5, 10, 15, 20 μg/mL), or ISL (0, 5, 10, 15, 20 μM) in time sequences (1, 5, and 20 days). Also, isoliquritigenin (ISL) was tested for comparison to those two biocompound extracts. Results: MTT assay results showed that all three compounds (ErLR, EdLR, and ISL) increased osteoblastic-cell proliferation in a concentration-dependent manner for one day. In addition, both ErLR and EdLR compounds elevated the osteoblast proliferation for 5 or 20 days. Extracellular ALP activity was also increased as ErLR, EdLR, and ISL concentration increased at 20 days, which implies the positive effect of Glycyrrhiza species on osteoblast mineralization. The bone-related marker mRNAs were upregulated in the ErLR-treated osteoblastic MC3T3-E1 cells for 20 days. Bone-specific transcription factor Runx2 gene expression was also elevated in the ErLR- and EdLR-treated osteoblastic MC3T3-E1 cells for 20 days. Conclusion: These results demonstrated that Glycyrrhiza uralensis extracts may be useful for preventing osteoporosis by increasing cell proliferation, ALP activity, and bone-marker gene expression in osteoblastic cells.
Go to : 
References
3. Appelman-Dijkstra NM, Papapoulos SE. Modulating bone resorption and bone formation in opposite directions in the treatment of postmenopausal psteoporosis. Drugs. 2015; 75(10):1049–1058.
5. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284(5411):143–147.

6. Seibel MJ. Biochemical markers of bone turnover. Part I: biochemistry and variability. Clin Biochem Rev. 2005; 26(4):97–122.
8. Asl MN, Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res. 2008; 22(6):709–724.
9. Yoon G, Jung YD, Cheon SH. Cytotoxic allyl retrochalcone from the roots of Glycyrrhiza inflata. Chem Pharm Bull (Tokyo). 2005; 53(6):694–695.

10. Shibata S. A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi. 2000; 120(10):849–862.
11. Hatano T, Fukuda T, Liu YZ, Noro T, Okuda T. Phenolic constituents of licorice. IV. Correlation of phenolic constituents and licorice specimens from various sources, and inhibitory effects of licorice extracts on xanthine oxidase and monoamine oxidase. Yakugaku Zasshi. 1991; 111(6):311–321.

12. Hayashi H, Hosono N, Kondo M, Hiraoka N, Ikeshiro Y, Shibano M, Kusano G, Yamamoto H, Tanaka T, Inoue K. Phylogenetic relationship of six Glycyrrhiza species based on rbcL sequences and chemical constituents. Biol Pharm Bull. 2000; 23(5):602–606.

13. Zeng L, Lou ZC, Zhang RY. Quality evaluation of Chinese licorice. Yao Xue Xue Bao. 1991; 26(10):788–793.
14. Choi HJ, Seon MR, Lim SS, Kim JS, Chun HS, Park JH. Hexane/ethanol extract of Glycyrrhiza uralensis licorice suppresses doxorubicin-induced apoptosis in H9c2 rat cardiac myoblasts. Exp Biol Med (Maywood). 2008; 233(12):1554–1560.
15. Park SY, Lim SS, Kim JK, Kang IJ, Kim JS, Lee C, Kim J, Park JH. Hexane-ethanol extract of Glycyrrhiza uralensis containing licoricidin inhibits the metastatic capacity of DU145 human prostate cancer cells. Br J Nutr. 2010; 104(9):1272–1282.
16. Seon MR, Park SY, Kwon SJ, Lim SS, Choi HJ, Park H, Lim DY, Kim JS, Lee CH, Kim J, Park JH. Hexane/ethanol extract of Glycyrrhiza uralensis and its active compound isoangustone A induce G1 cycle arrest in DU145 human prostate and 4T1 murine mammary cancer cells. J Nutr Biochem. 2012; 23(1):85–92.

17. Vaya J, Belinky PA, Aviram M. Antioxidant constituents from licorice roots: isolation, structure elucidation and antioxidative capacity toward LDL oxidation. Free Radic Biol Med. 1997; 23(2):302–313.

19. Choi EM. Liquiritigenin isolated from Glycyrrhiza uralensis stimulates osteoblast function in osteoblastic MC3T3-E1 cells. Int Immunopharmacol. 2012; 12(1):139–143.

20. Cho YE, Kwun IS. Zinc upregulates bone-specific transcription factor Runx2 expression via BMP-2 signaling and Smad-1 phosphorylation in osteoblasts. J Nutr Health. 2018; 51(1):23–30.

21. Cho YE, Alcantara E, Kumaran S, Son KH, Sohn HY, Lee JH, Choi CS, Ha TY, Kwun IS. Red yeast rice stimulates osteoblast proliferation and increases alkaline phosphatase activity in MC3T3-E1 cells. Nutr Res. 2010; 30(7):501–510.

22. Che CT, Wong MS, Lam CW. Natural products from Chinese medicines with potential benefits to bone health. Molecules. 2016; 21(3):239.

23. Aubin JE, Liu F, Malaval L, Gupta AK. Osteoblast and chondroblast differentiation. Bone. 1995; 17(2 Suppl):77S–83S.

24. Stein GS, Lian JB. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr Rev. 1993; 14(4):424–442.

25. Bellows CG, Aubin JE, Heersche JN. Initiation and progression of mineralization of bone nodules formed in vitro: the role of alkaline phosphatase and organic phosphate. Bone Miner. 1991; 14(1):27–40.

26. Komori T. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem. 2006; 99(5):1233–1239.

27. Liu W, Toyosawa S, Furuichi T, Kanatani N, Yoshida C, Liu Y, Himeno M, Narai S, Yamaguchi A, Komori T. Overexpression of Cbfa1 in osteoblasts inhibits osteoblast maturation and causes osteopenia with multiple fractures. J Cell Biol. 2001; 155(1):157–166.

28. Banerjee C, McCabe LR, Choi JY, Hiebert SW, Stein JL, Stein GS, Lian JB. Runt homology domain proteins in osteoblast differentiation: AML3/CBFA1 is a major component of a bone-specific complex. J Cell Biochem. 1997; 66(1):1–8.

Go to : 
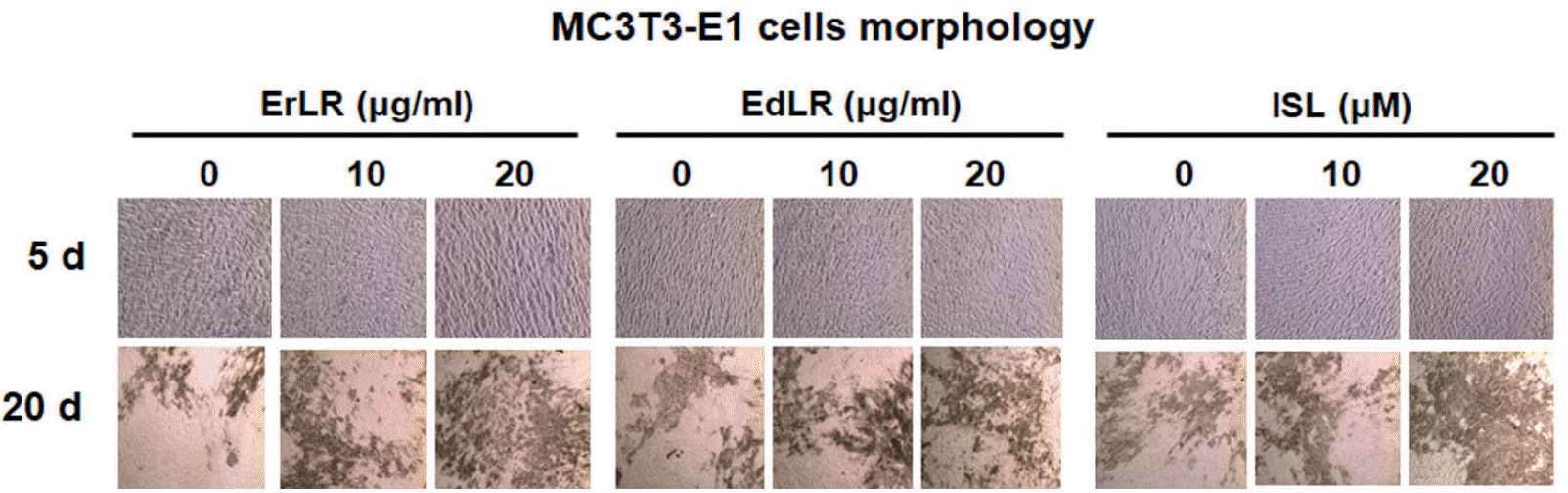 | Fig. 1.Morphological characteristics of osteoblastic MC3T3-E1 cells treated with various ErLR, EdLR, and ISL compounds. Photographs were taken with a phase-contrast microscope at 100 × magnification. |
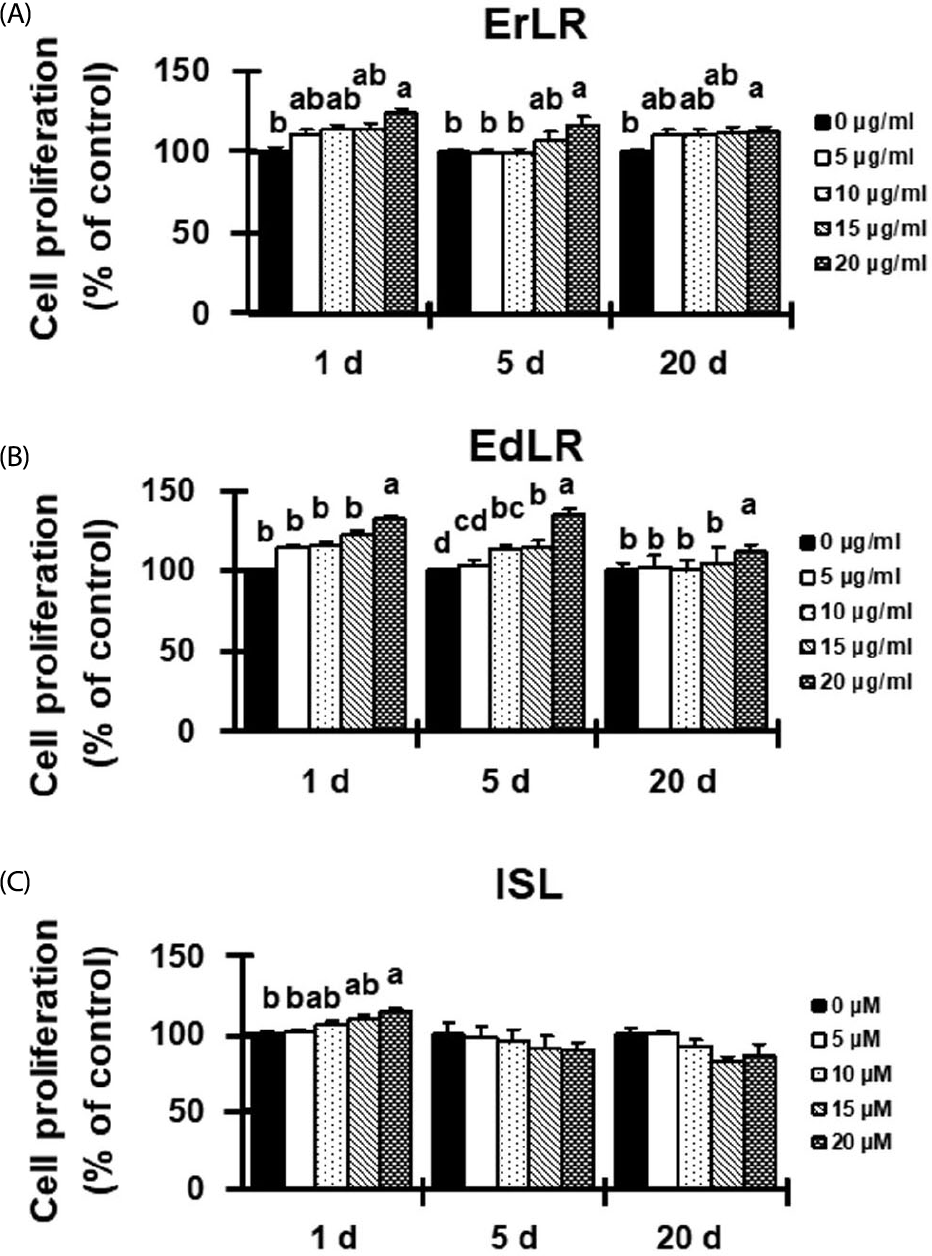 | Fig. 2.Effect of ErLR (A), EdLR (B), and ISL (C) compounds on the proliferation of osteoblastic MC3T3-E1 cells. Significant differences between various treatments were found by one-way ANOVA. Labelled characters without a common letter represent significant differences from the other group(s). The values are presented as % of control at 0 h. |
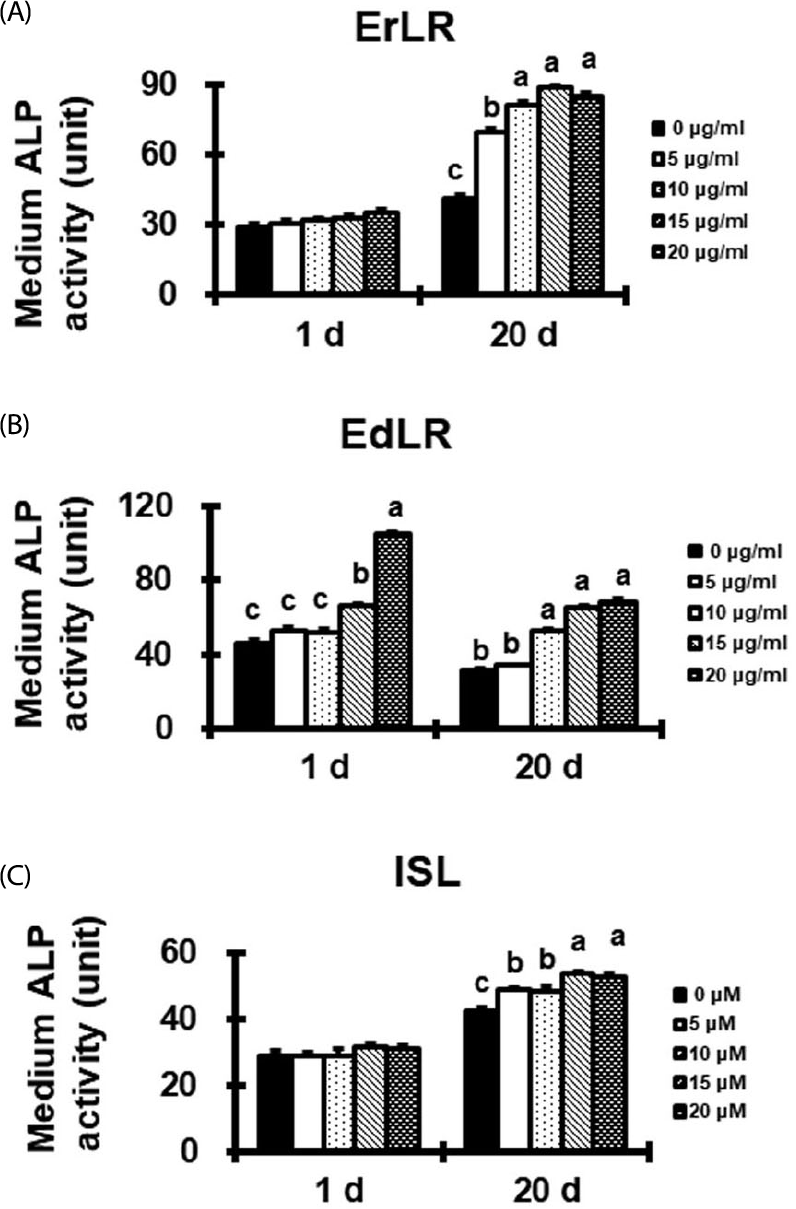 | Fig. 3.Effect of ErLR (A), EdLR (B), and ISL (C) compounds on the medium ALP activity of osteoblastic MC3T3-E1 cells. Significant difference between treatments were found by one-way ANOVA. Labelled characters without a common letter represent significant differences from the other group(s) at p < 0.05. |
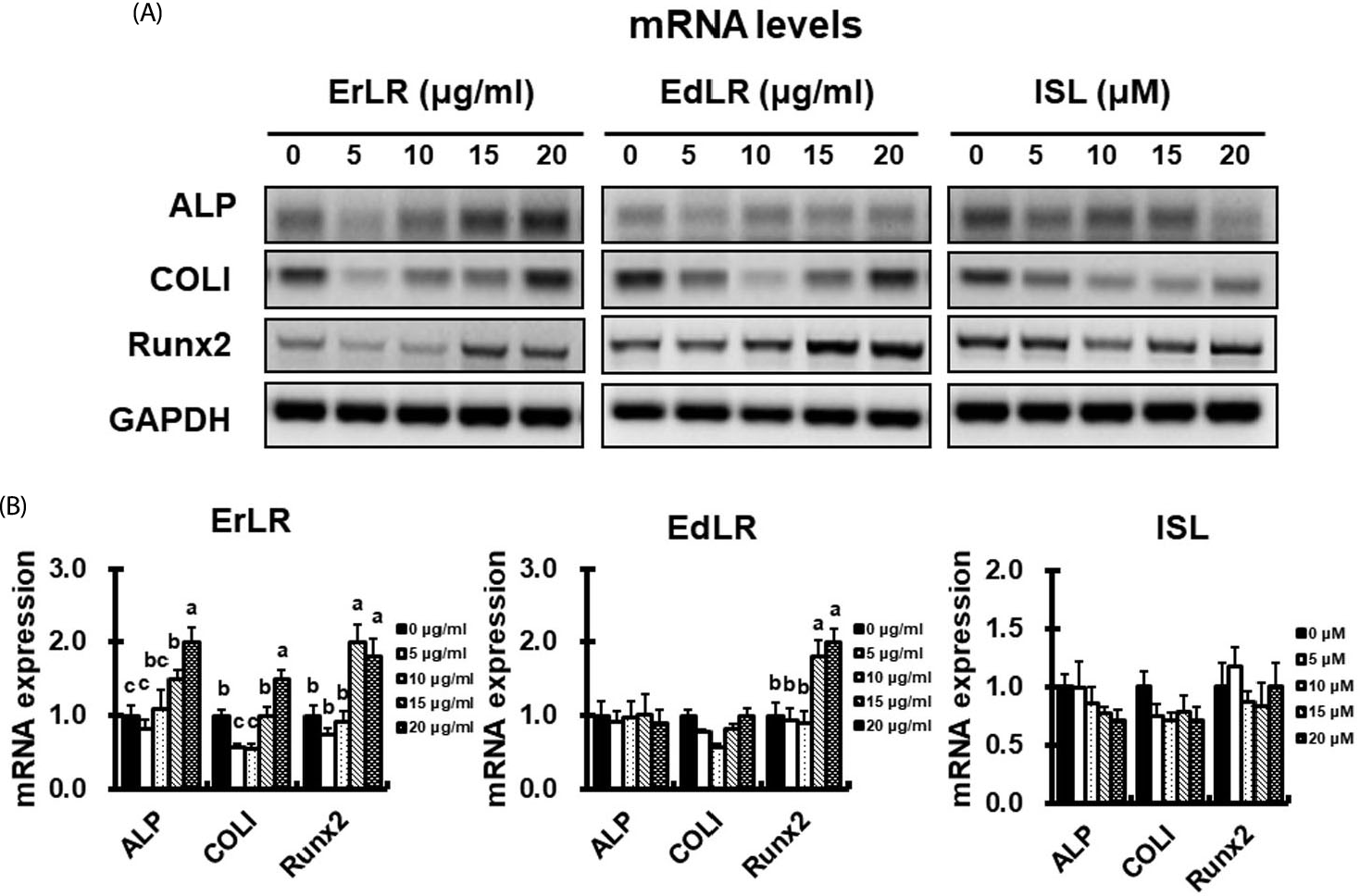 | Fig. 4.Gene expression of bone-marker genes (ALP and COLI) and transcription factor Runx2 in osteoblastic MC3T3-E1 cells treated with various ErLR, EdLR, and ISL compounds for 20 days. (A-B) mRNA transcription level of bone markers was measured using quantitative real-time PCR (qRT-PCR). Significant difference between treatments were found by one-way ANOVA. Labelled characters without a common letter represent significant differences from the other group(s). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download