Abstract
Background
Vitamin D plays an important role in the immune response against infection. The purpose of the present study was to investigate the influence of vitamin D deficiency on the progression of otitis media (OM) using an experimental rat model.
Methods
Four-week-old male Sprague-Dawley rats (n=72) were divided into two groups based on their diet: a control diet group (n=36) and a vitamin D-deficient diet group (n=36). After 8 weeks of diet, experimental OM was induced by inoculation of non-typeable Haemophilus influenzae in the middle ear cavity. The rats were evaluated with otomicroscopy to determine the inflammation in the middle ear mucosa on days 1, 2, 4, 7, and 14 post-inoculation. Bullae from sacrificed rats were collected and analyzed histologically.
Results
The middle ear mucosa from rats with vitamin D deficiency showed a significantly higher thickness than that of controls during the course of OM. The maximum mucosal thickness was 56.0±9.1 µm in the vitamin D deficiency group, and 43.9±9.8 µm in the control group, although there was no significant difference in the tympanic membrane score between the two groups evaluated with otomicroscopy. An immunohistochemical study showed increased expression of interleukin 6 (IL-6) and tumor necrosis factor α in rats manifesting vitamin D deficiency and decreased expression of IL-10 compared with controls.
Vitamin D is well known for its crucial role in bone and mineral metabolism. Besides, its role in immune response to infection has been an interesting topic in vitamin D research [123]. In the pre-antibiotic era, sunlight exposure or cod liver oil, which presumably raised the vitamin D level of individuals, was adopted as an effective treatment for tuberculosis infection [3]. Recently, accumulating evidence based on scientific studies suggests that vitamin D plays an important role in the immune system. Vitamin D receptor is expressed in most cells of the immune system and its genetic polymorphisms have been associated with susceptibility to infection [456]. The vitamin D-activating enzyme, 25-hydroxyvitamin D (25(OH)D)-1α-hydroxylase, is also expressed in immune cells including macrophages, dendritic cells, and T and B lymphocytes [4]. In addition, the mechanisms underlying the immunoregulatory function of vitamin D including regulation of cytokine profiles or antimicrobial peptides have been reported [123].
Otitis media (OM) is one of the most common inflammatory diseases associated with childhood. Approximately 80% of children report at least one episode of acute OM and more than 40% experience three or more episodes by the age of 3 years [7]. OM is caused by the interaction between multiple factors including Eustachian tube dysfunction, viral and bacterial infection, exposure to smoke, and impaired host immunity [89]. Pathologically, OM is characterized by transformation and hyperplasia of the middle ear mucosa following infiltration of various inflammatory cells [10]. A variety of animal models have been developed to explore and understand the pathophysiological mechanisms of OM [11], although the mechanism underlying hyperplastic changes has not been fully identified.
Several clinical studies reported a significant association between vitamin D status and OM [12131415]. However, little is known about the role of vitamin D status in the pathophysiology of OM. In the present study, we investigated the influence of vitamin D deficiency on the progression of OM using an experimental rat model.
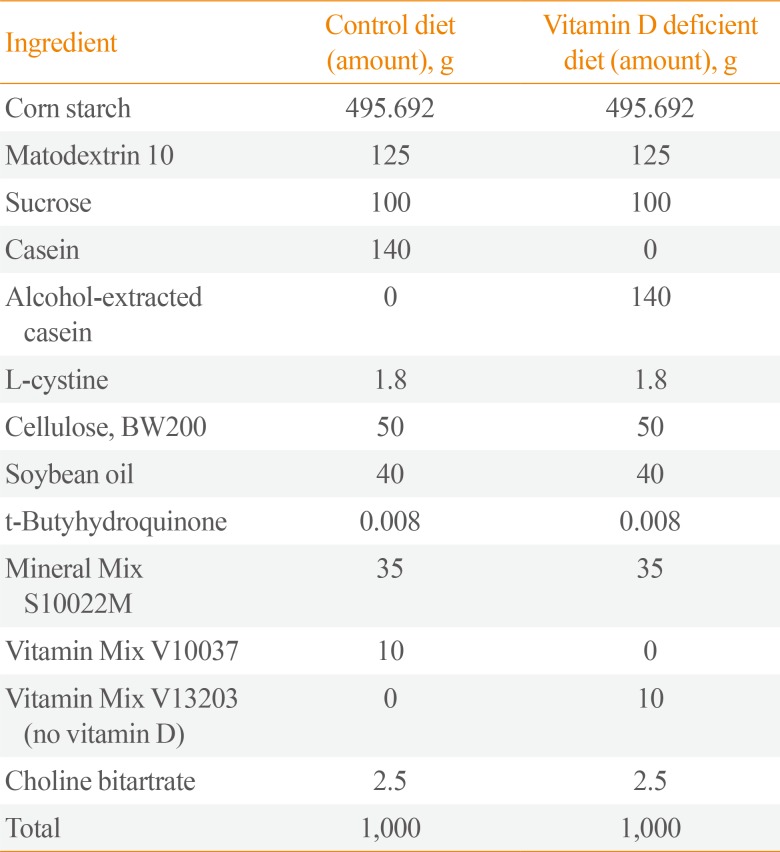
A total of 72 male Sprague-Dawley rats (Harlan CPB, Horst, the Netherlands) aged 4 weeks, and each weighing 200 to 230 g at the beginning of the study were used. The rats underwent 1-week acclimatization to the animal facility prior to study. The breeding room was maintained at a temperature of 21℃ to 23℃ with 40% to 60% humidity and a 12-hour light/darkness cycle. The rats were divided into two groups based on their diet (Rearch Diets Inc., New Brunswick, NJ, USA): control diet group (n=36) and vitamin D-deficient diet group (n=36) as shown in Table 1. Starting with day 1 of the experiment, the rats were fed a control diet or a vitamin D-deficient diet for 8 weeks. Subsequently, experimental OM was induced by inoculation of nontypeable Haemophilus influenzae (NTHi) 3,655 in the middle ear of the rats. Rats were sacrificed on days 1, 2, 4, 7, and 14 post-inoculation for evaluation. All care and experiments were performed in accordance with the regulations of the Animal Research Institute of Medical Science, Dongguk University, and the study protocol was approved by the Animal Institutional Review Board (AIRB no. 2016-02114).
NTHi 3655 was preserved in a deep freezer at −80℃. It was used to induce OM with effusion. The bacteria were streaked onto BD BBL™ chocolate II agar dish (prod. no. 221169, Becton Dickinson, Franklin Lakes, NJ, USA). The dish was incubated overnight at 37℃ and 5% CO2. Two colonies were selected and blended with 25 mL Brain Heart Infusion Broth (prod. no. B9500, Teknova Inc., Burlington, ON, Canada) and 1 mL of Remel™ Fildes enrichment (prod. no. R45037, Thermo Fisher Scientific, Waltham, MA, USA). The mixture was incubated overnight at 37℃ in the rotator, and centrifuged at 1,000 ×g for 5 minutes. Finally, the solution contained a bacterial suspension (105 cells/mL).
The tympanic membrane of rats was observed for abnormalities such as middle ear effusion, using otomicroscopy. The anesthetic solution used for the animals contained 0.1 mL/kg of ketamine (Bayer, Leverkusen, Germany) and 0.1 mL/kg of Zoletile (Virbac, Carros, France). Under anesthesia, the rat was held in the supine position. Anterior neck was disinfected with betadine and a vertical incision was made. The soft tissue was dissected and both bullae were exposed by pushing aside the strap muscle and the submandibular gland. A 25 G needle tip was used to pierce a hole in the bulla, and 0.05 mL of bacterial suspension was injected into the hole.
The anesthetic solution was injected on days 1, 2, 4, 7, and 14 post-inoculation to obtain a picture of the tympanic membrane and to monitor the progress of OM. Inflammation of the middle ear mucosa and middle ear effusion in all the rats was ensured using a digital microscope-USB (Dino-Lite, New Taipei City, Taiwan). Scoring of tympanic membrane was made based on the otomicroscopic findings (0: normal; 1: 1/2 or less exudate, congestion; 2: more than 1/2 exudate, severe congestion; 3: high-pitched whole effervescent fluid, severe congestion).
The rats were anesthetized and 0.5 to 1 mL of blood was drawn by cardiac puncture at the time of sacrifice. Blood samples were collected in BD Microtainer SST™ (prod. no. REF 365967, Becton Dickinson) and centrifuged. The collected serum samples were left in a −80℃ deep freezer. For determination of vitamin D levels, 25-OH vitamin D total ELISA kits (ALPCO, Salem, NH, USA) were used. Each vitamin D concentration was determined using spectrophotometry.
The 72 bullae were fixed in 4% neutral buffered formalin for 24 hours, decalcified for over 3 days, using a decalcifying solution (prod. no. D0818-1L, Sigma-Aldrich, St. Louis, MO, USA). The tissue was embedded in paraffin and sliced to a thickness of 4 µm on a microtome (prod. no. RM2235, Leica, Nussloch, Germany). For the analysis of mucosal thickness, hematoxylin (prod. no. HHS32, Sigma-Aldrich) and eosin Y (prod. no. E6003, Sigma-Aldrich) (H&E) stains were used on the prepared slides. Photographs of each slide at 400× magnification were obtained. To determine the expression of cytokines and antimicrobial peptides, all slides were immunostained with interleukin 6 (IL-6) (prod. no. orb303667, Biorbyt Ltd., Cambridge, UK), tumor necrosis factor α (TNF-α) (prod. no. ab6671, Abcam, Cambridge, MA, USA), IL-10 (prod. no. P29456, Cloud-Clone Corp., Huston, TX, USA), cathelicidin-related antimicrobial peptide (CRAMP) (prod. no. sc-374218, Santa Cruz Biotechnology Inc., Dallas, TX, USA), and β-defensin 2 (prod. no. 251659, Abbiotec LLC, San Diego, CA, USA) and coupled with 3, 3′-diaminobenzidine (prod. no. K3468, Dako North America Inc., Carpinteria, CA, USA) for color development. We obtained microscopic images and photographs at 400× magnification of each slide using Olympus BX53F (Olympus, Tokyo, Japan). All the detected antibodies were collected using LEICA Qwin V3 (Leica Microsystems Imaging Solutions Ltd., Cambridge, UK) digital image processing and analysis software.
A summary of otomicroscopic findings of OM in the control and vitamin D deficiency groups is presented in Fig. 1A. Inflammation of each middle ear was graded according to the otomicroscopic findings. The mean value of the tympanic membrane scores in both groups are listed in Fig. 1B. The maximum increase in OM with effusion in both groups occurred on days 1 and 2, which decreased subsequently. After day 7, the tympanic membrane has almost returned to normal. However, there was no statistical difference of the scores between control group and vitamin D deficiency group.
Bullae from control and vitamin D deficiency groups were stained with H&E (Fig. 2A). The mucosal thickness of both groups increased gradually from day 1. The middle ear mucosal thickness of the vitamin D deficiency group was significantly higher than that of the control group from day 2 to day 7 (Fig. 2B). On day 2, the maximum thickness was 56.0±9.1 µm in the vitamin D deficiency group and 43.9±9.8 µm in the control group, which represents 28.2% higher thickness in the vitamin D deficiency group. On days 4 and 7, the mucosal thickness of the vitamin D deficiency group was 47% to 50% higher than that of the control group. Most of the middle ear mucosa was restored by day 14.
The serum 25(OH)D concentration was 75.19±43.43 ng/mL in the control group and 17.43±15.10 ng/mL in the vitamin D deficiency group (P<0.05) (Fig. 3).
Immunohistochemical staining showed that proinflammatory cytokine IL-6 detection area gradually increased from day 0 (0.27%±0.10%) to day 7 (3.29%±1.21%) in the vitamin D deficiency group, while its detection area was slightly altered between day 1 and 7 in the control group. This difference of the detection area made a statistical significance on day 7 (P<0.05) (Fig. 4A, B). TNF-α detection areas also showed a gradual increase from day 0 (0.29%±0.22%) to day 4 (3.12%±1.37%) in the vitamin D deficiency group, while its detection area remained almost unchanged between day 1 and 4 in the control group. There was a statistically significant difference of the TNF-α detection area between the control group and the vitamin D deficiency group on day 2 (P<0.05) and day 4 (P<0.005) (Fig. 4C, D). Anti-inflammatory cytokine IL-10 detection increased gradually from day 0 (3.13%±1.23%) to day 7 (12.50%±4.11%) in the control group. Its expression also increased from day 0 (3.02%±1.02%) to day 2 (8.83%±2.49%), but decreased from day 4 (8.83%±3.93%) to day 14 (7.28%±2.30%) in the vitamin D deficiency group, which made a statistically significant difference on day 7 (P<0.05) and day 14 (P<0.05) (Fig. 4E, F).
The expression of innate immune defense factor CRAMP was increased in the control group compared with the vitamin D deficiency group on day 1, but its expression was decreased in the control group compared with the vitamin D deficiency group on day 2 (Supplemental Fig. S1A, B). β-Defensin 2 was highly expressed in the control group than in the vitamin D deficiency group on day 7 (Supplemental Fig. S1C, D). Most of the CRAMP and β-defensin 2 expression in both groups was recovered by day 7.
Several clinical studies indicate that low vitamin D status is significantly associated with a high risk or severity of OM [12131415]. A recent case-control study by Cayir et al. [12] showed that the mean serum 25(OH)D level was significantly lower in children with recurrent OM compared with controls (28.5 nmol/L vs. 72.9 nmol/L). Another case-control study by Walker et al. [13] also showed that children with a higher serum 25(OH)D level carried a lower risk of chronic OM with effusion (odds ratio, 0.86 per 10 nmol/L; 95% confidence interval, 0.77 to 0.97). In a randomized controlled trial, administration of vitamin D 1,000 IU per day for 4 months significantly reduced the risk of uncomplicated acute OM in children with a history of recurrent acute OM (hazard ratio, 0.23; 95% confidence interval, 0.12 to 0.46) [14]. In addition, a meta-analysis of five clinical studies involving 16,689 subjects concluded that the lower vitamin D level might play an important role in increasing the occurrence of acute OM [15].
Despite the aforementioned clinical evidence showing a significant association between vitamin D status and OM, little is known about the pathophysiological changes underlying this association. The middle ear mucosa normally consists of a simple squamous epithelium ranging from 15 to 20 µm in thickness. However, bacterial infection or trauma can trigger infiltration of inflammatory cells leading to mucosal growth and proliferation into a pseudostratified columnar epithelium that may exceed 1,000 µm in thickness [10]. In the present study, the rats showed a thickening of middle ear mucosa after induction of experimental OM. We found that rats with vitamin D deficiency showed significantly higher mucosal thickness than controls during the course of OM, particularly from day 2 to 7. This finding indicates that vitamin D deficiency might be a factor that exacerbates the pathological changes of OM.
Vitamin D appears to influence the host immune system via multiple ways. One of the most important mechanisms involves T-cell–mediated immunity and cytokine production. By activating the vitamin D receptors in T-cell, vitamin D promotes the proliferation of type 2 helper T (TH2) cells and related cytokine production while suppressing type 1 helper T (TH1) cell proliferation and associated cytokine expression [123]. TH1 cytokines, which include IL-2, interferon γ, and TNF-α, are generally considered proinflammatory. By contrast, TH2 cytokines including IL-4, IL-5, and IL-10 appear to exhibit anti-inflammatory effects [2]. Therefore, optimal vitamin D status leads to a healthy and tolerogenic immune response, whereas vitamin D deficiency may lead to a more proinflammatory state. Vitamin D also appears to promote regulatory T-cell production, which limits the extent of tissue damage [23]. In addition, it was shown that vitamin D inhibits the production of proinflammatory cytokines in the respiratory epithelium by modulating the nuclear factor κB pathway [1617]. In the present study, our results also showed that the expression of IL-6 and TNF-α in the middle ear mucosa was increased, while IL-10 expression was decreased in the vitamin D-deficient rats, which indicates that vitamin D deficiency may alter the host immune response to a more proinflammatory state in OM.
It was also suggested that vitamin D upregulated the expression of antimicrobial peptide in phagocytes and epithelial cells. The vitamin D receptor-binding site, the so-called vitamin D response element, was discovered in the promoter regions of the genes encoding antimicrobial peptides such as cathelicidin and β-defensin 2, which have a lethal effect on microsomes [181920]. However, this mechanism was shown to be conserved in only humans and primates, but not in nonprimate mammals including mice and rats [1920]. Immunohistochemistry of our study showed an inconsistent result. The expression of CRAMP on day 1 after induction of OM was decreased in the vitamin D deficiency group compared with controls, but its expression on day 2 was increased in the vitamin D deficiency group. The expression of β-defensin 2 was decreased in the vitamin D deficiency group compared with controls on day 7. In addition, other antimicrobial factors such as reactive oxygen species and nitric oxide synthase were also known to be stimulated by vitamin D [321].
The present study has a few limitations. First, the role of vitamin D in the OM pathophysiology was investigated only in rats with vitamin D deficiency. To elucidate the effects of vitamin D on the development or progression of OM, further studies investigating the role of vitamin D supplementation are needed. Second, the altered expression of cytokines in the middle ear mucosa resulting from vitamin D deficiency was demonstrated immunohistochemically. Serum levels of cytokines were not investigated in the present study. Therefore, it is not clear whether the role of vitamin D in the progression of OM is mediated via local or systemic mechanisms.
In conclusion, our study showed that pathological changes in the middle ear mucosa derived from OM were more severe in rats with vitamin D deficiency compared with controls, although the duration of OM was not different between the two groups. Our results also showed that vitamin D deficiency is associated with a proinflammatory state due to the increased production of proinflammatory cytokines and decreased expression of anti-inflammatory cytokines. Based on these findings, we suggest that vitamin D deficiency may exacerbate the pathophysiological changes of OM via altered cytokine production. Therefore, maintenance of vitamin D status in the optimal range may be critical to successful clinical management of OM. The influence of vitamin D supplementation on the pathophysiology of OM requires additional investigation.
ACKNOWLEDGMENTS
This research was funded by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2015R1C1A1A01054333).
Notes
References
1. Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013; 5:2502–2521. PMID: 23857223.

2. Gunville CF, Mourani PM, Ginde AA. The role of vitamin D in prevention and treatment of infection. Inflamm Allergy Drug Targets. 2013; 12:239–245. PMID: 23782205.

3. Borella E, Nesher G, Israeli E, Shoenfeld Y. Vitamin D: a new anti-infective agent. Ann N Y Acad Sci. 2014; 1317:76–83. PMID: 24593793.

4. Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008; 29:726–776. PMID: 18694980.

5. Rathored J, Sharma SK, Singh B, Banavaliker JN, Sreenivas V, Srivastava AK, et al. Risk and outcome of multidrug-resistant tuberculosis: vitamin D receptor polymorphisms and serum 25(OH)D. Int J Tuberc Lung Dis. 2012; 16:1522–1528. PMID: 22990231.

6. Aslan S, Akil I, Aslan G, Onay H, Ozyut BC, Ozkinay F. Vitamin D receptor gene polymorphism in children with urinary tract infection. Pediatr Nephrol. 2012; 27:417–421. PMID: 21947233.

7. Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989; 160:83–94. PMID: 2732519.

8. Mittal R, Kodiyan J, Gerring R, Mathee K, Li JD, Grati M, et al. Role of innate immunity in the pathogenesis of otitis media. Int J Infect Dis. 2014; 29:259–267. PMID: 25447732.

9. Cho CG, Gong SH, Kim HB, Song JJ, Park JH, Lim YS, et al. Role of group 3 innate lymphoid cells during experimental otitis media in a rat model. Int J Pediatr Otorhinolaryngol. 2016; 88:146–152. PMID: 27497403.

10. Lim DJ, Birck H. Ultrastructural pathology of the middle ear mucosa in serous otitis media. Ann Otol Rhinol Laryngol. 1971; 80:838–853. PMID: 5127754.

11. Cho CG. Animal models of otitis media. Korean J Otorhinolaryngol-Head Neck Surg. 2015; 58:371–377.

12. Cayir A, Turan MI, Ozkan O, Cayir Y, Kaya A, Davutoglu S, et al. Serum vitamin D levels in children with recurrent otitis media. Eur Arch Otorhinolaryngol. 2014; 271:689–693. PMID: 23543299.

13. Walker RE, Bartley J, Camargo CA Jr, Flint D, Thompson JM, Mitchell EA. Higher serum 25(OH)D concentration is associated with lower risk of chronic otitis media with effusion: a case-control study. Acta Paediatr. 2017; 106:1487–1492. PMID: 28477429.

14. Marchisio P, Consonni D, Baggi E, Zampiero A, Bianchini S, Terranova L, et al. Vitamin D supplementation reduces the risk of acute otitis media in otitis-prone children. Pediatr Infect Dis J. 2013; 32:1055–1060. PMID: 23694840.

15. Li HB, Tai XH, Sang YH, Jia JP, Xu ZM, Cui XF, et al. Association between vitamin D and development of otitis media: a PRISMA-compliant meta-analysis and systematic review. Medicine (Baltimore). 2016; 95:e4739. PMID: 27749530.
16. Hansdottir S, Monick MM, Lovan N, Powers L, Gerke A, Hunninghake GW. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol. 2010; 184:965–974. PMID: 20008294.
17. McNally P, Coughlan C, Bergsson G, Doyle M, Taggart C, Adorini L, et al. Vitamin D receptor agonists inhibit proinflammatory cytokine production from the respiratory epithelium in cystic fibrosis. J Cyst Fibros. 2011; 10:428–434. PMID: 21784717.

18. Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004; 173:2909–2912. PMID: 15322146.

19. Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005; 19:1067–1077. PMID: 15985530.
20. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006; 311:1770–1773. PMID: 16497887.

21. Rockett KA, Brookes R, Udalova I, Vidal V, Hill AV, Kwiatkowski D. 1,25-DihydroxyvitaminD3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect Immun. 1998; 66:5314–5321. PMID: 9784538.
SUPPLEMENTARY MATERIAL
Supplemental Fig. S1
Immunohistochemical staining and the average detection area (%) of (A, B) cathelicidin-related antimicrobial peptide (CRAMP) and (C, D) β-defensin 2 for bacterially induced middle ear mucosa. aP<0.05; bP<0.005.
Fig. 1
(A) Serial otomicroscopic images of middle ear in bacterially induced otitis media were taken for 14 days (×90 magnification). (B) The mean value of the tympanic membrane scores in the control group and vitamin D deficiency group (0: normal; 1: 1/2 or less exudate; congestion; 2: more than 1/2 exudate, severe congestion; 3: high-pitched whole effervescent fluid, severe congestion).
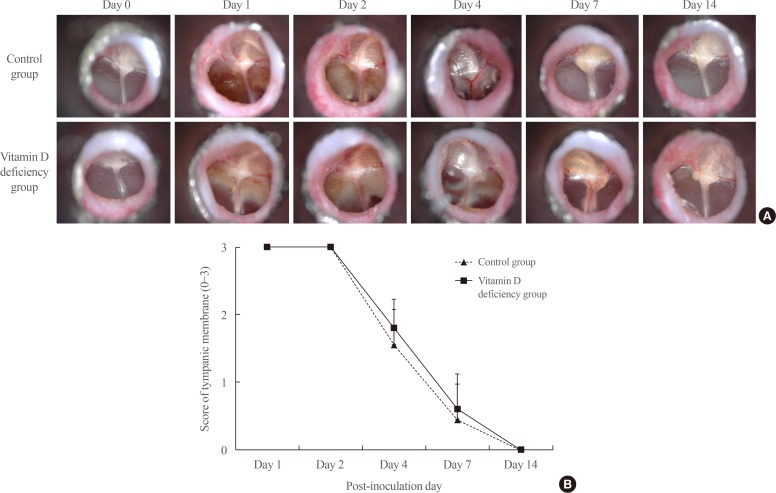
Fig. 2
(A) H&E stain of middle ear mucosa (×200 magnification). (B) Middle ear mucosa thickness. aP<0.005.
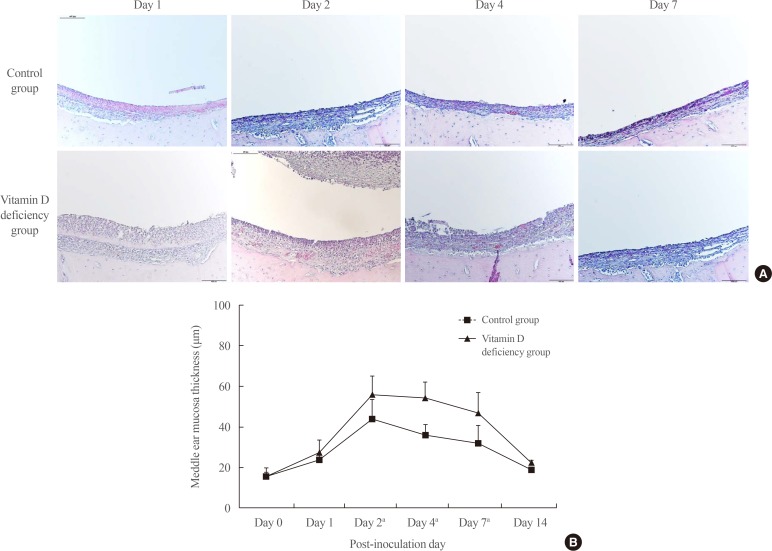
Fig. 3
Serum 25-hydroxyvitamin D (25(OH)D) levels in the control group and the vitamin D deficiency group. aP<0.05.
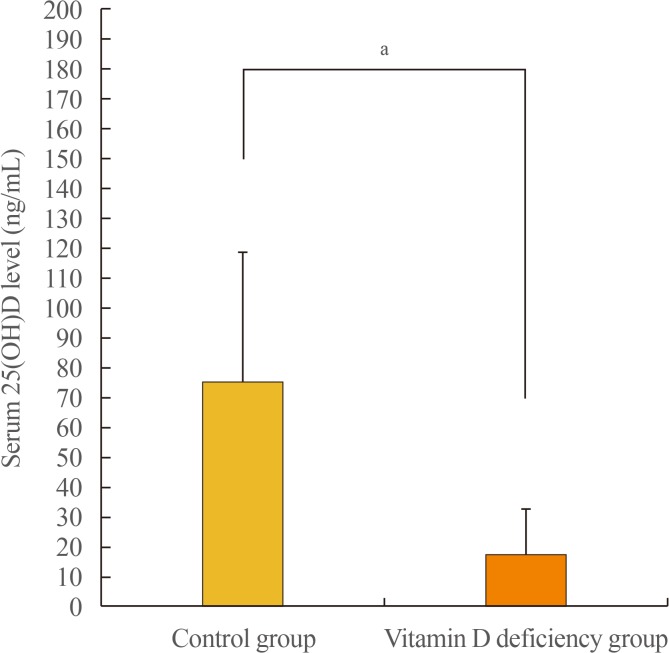
Fig. 4
Immunohistochemical stain and the average detection area (%) of (A, B) interleukin 6 (IL-6), (C, D) tumor necrosis factor α (TNF-α), and (E, F) IL-10 for bacterially induced middle ear mucosa. aP<0.05; bP<0.005.
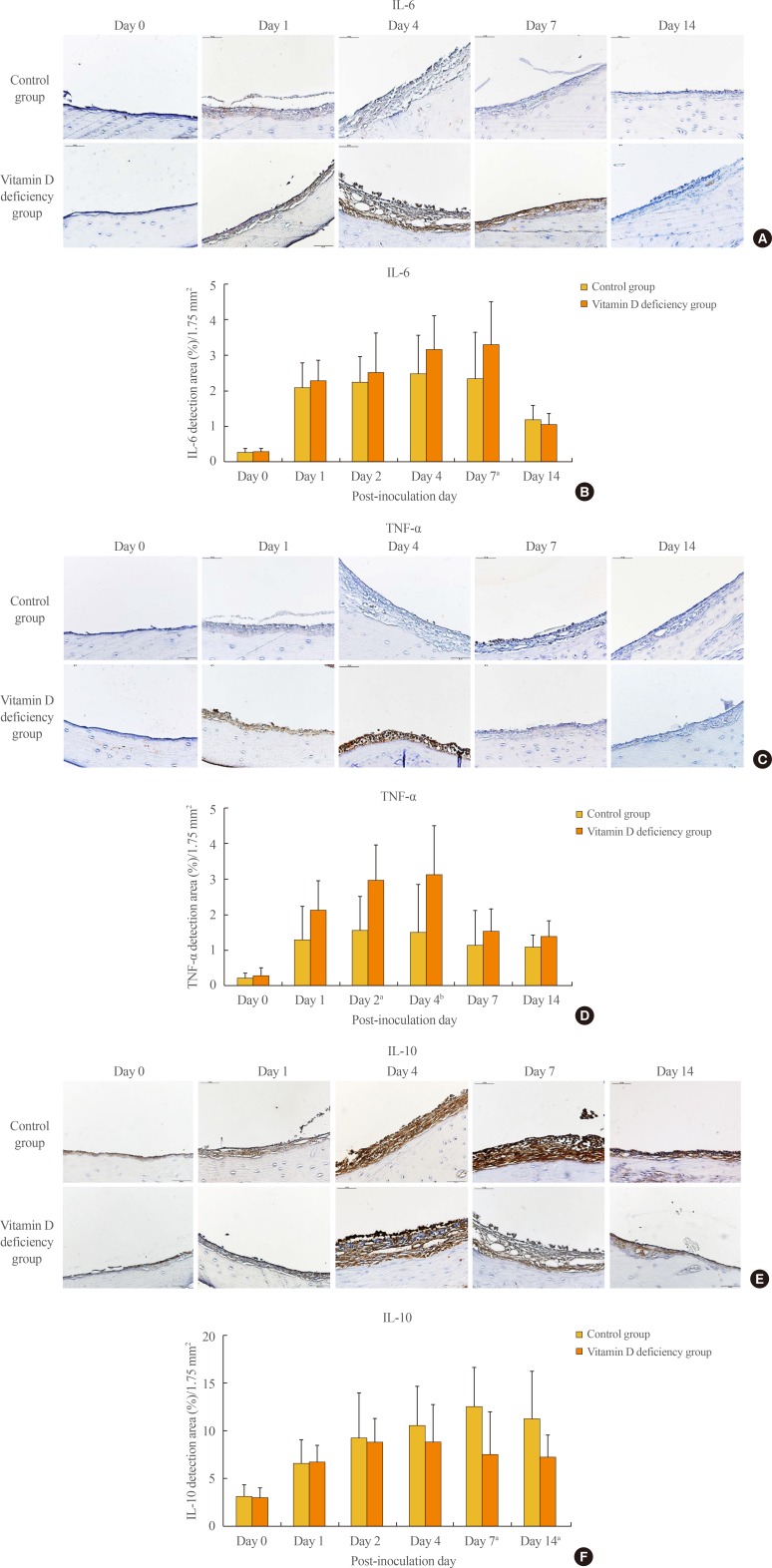
Table 1
Contents of Diet Provided to Experimental Rats




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download