Abstract
Objective
The Japan Society of Gynecologic Oncology (JSGO) published the first practice guideline for endometrial cancer in 2006. The JSGO guideline evaluation committee assessed the effect of this guideline introduction on clinical practice and patient outcome using data provided by the Japan Society of Obstetrics and Gynecology (JSOG) cancer registration system.
Methods
Data of patients with endometrial cancer registered between 2000 and 2012 were analyzed, and epidemiological and clinical trends were assessed. The influence of guideline introduction on survival was determined by analyzing data of patients registered between 2004 and 2009 using competing risk model.
Results
In total, 65,241 cases of endometrial cancer were registered. Total number of patients registered each year increased about 3 times in the analyzed period, and the proportion of older patients with type II endometrial cancer rapidly increased. The frequency of lymphadenectomy had decreased not only among the low-recurrence risk group but also among the intermediate- or high-recurrence risk group. Adjuvant therapy was integrated into chemotherapy (p<0.001). Overall survival did not significantly differ before and after the guideline introduction (hazard ratio [HR]=0.891; p=0.160). Additional analyses revealed patients receiving adjuvant chemotherapy showed better prognosis than those receiving adjuvant radiation therapy when limited to stage I or II (HR=0.598; p=0.003).
References
2. Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H, et al. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015; 45:884–91.

3. Nagase S, Katabuchi H, Hiura M, Sakuragi N, Aoki Y, Kigawa J, et al. Evidence-based guidelines for treatment of uterine body neoplasm in Japan: Japan Society of Gynecologic Oncology (JSGO) 2009 edition. Int J Clin Oncol. 2010; 15:531–42.

4. Ebina Y, Katabuchi H, Mikami M, Nagase S, Yaegashi N, Udagawa Y, et al. Japan Society of Gynecologic Oncology guidelines 2013 for the treatment of uterine body neoplasms. Int J Clin Oncol. 2016; 21:419–34.

5. McCluggage WG. Malignant biphasic uterine tumours: carcinosarcomas or metaplastic carcinomas? J Clin Pathol. 2002; 55:321–5.
7. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999; 94:496–509.

8. Shepherd JH. Revised FIGO staging for gynaecological cancer. Br J Obstet Gynaecol. 1989; 96:889–92.

9. Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009; 105:109.

10. Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016; 387:1094–108.

12. Brinton LA, Felix AS, McMeekin DS, Creasman WT, Sherman ME, Mutch D, et al. Etiologic heterogeneity in endometrial cancer: evidence from a Gynecologic Oncology Group trial. Gynecol Oncol. 2013; 129:277–84.

13. Cragun JM, Havrilesky LJ, Calingaert B, Synan I, Secord AA, Soper JT, et al. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J Clin Oncol. 2005; 23:3668–75.

14. Candiani GB, Belloni C, Maggi R, Colombo G, Frigoli A, Carinelli SG. Evaluation of different surgical approaches in the treatment of endometrial cancer at FIGO stage I. Gynecol Oncol. 1990; 37:6–8.

15. Trimble EL, Kosary C, Park RC. Lymph node sampling and survival in endometrial cancer. Gynecol Oncol. 1998; 71:340–3.

16. Bar-Am A, Ron IG, Kuperminc M, Gal I, Jaffa A, Kovner F, et al. The role of routine pelvic lymph node sampling in patients with stage I endometrial carcinoma: second thoughts. Acta Obstet Gynecol Scand. 1998; 77:347–50.

17. Sartori E, Gadducci A, Landoni F, Lissoni A, Maggino T, Zola P, et al. Clinical behavior of 203 stage II endometrial cancer cases: the impact of primary surgical approach and of adjuvant radiation therapy. Int J Gynecol Cancer. 2001; 11:430–7.

18. Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2006; 24:36–44.

19. Takayama S, Monma Y, Tsubota-Utsugi M, Nagase S, Tsubono Y, Numata T, et al. Food intake and the risk of endometrial endometrioid adenocarcinoma in Japanese women. Nutr Cancer. 2013; 65:954–60.

20. Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002; 11:1531–43.
21. Dunn JE. Cancer epidemiology in populations of the United States–with emphasis on Hawaii and California–and Japan. Cancer Res. 1975; 35:3240–5.
22. Ueda SM, Kapp DS, Cheung MK, Shin JY, Osann K, Husain A, et al. Trends in demographic and clinical characteristics in women diagnosed with corpus cancer and their potential impact on the increasing number of deaths. Am J Obstet Gynecol. 2008; 198:218. e1–6.

23. Melamed A, Rauh-Hain JA, Clemmer JT, Diver EJ, Hall TR, Clark RM, et al. Changing trends in lymphadenectomy for endometrioid adenocarcinoma of the endometrium. Obstet Gynecol. 2015; 126:815–22.

24. ASTEC study groupKitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet. 2009; 373:125–36.

25. Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008; 100:1707–16.

26. Todo Y, Kato H, Kaneuchi M, Watari H, Takeda M, Sakuragi N. Survival effect of paraaortic lymphadenectomy in endometrial cancer (SEPAL study): a retrospective cohort analysis. Lancet. 2010; 375:1165–72.

27. National Comprehensive Cancer Network (US). NCCN Clinical Practice Guidelines in Oncology. Uterine neoplasms, version 2. 2016 [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;2016. [cited 2016 Oct 28]. Available from:. https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf.
28. Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: diagnosis, treatment and follow-up. Int J Gynecol Cancer. 2016; 26:2–30.

29. Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, et al. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013; 24(Suppl 6):vi33–8.

30. Lee SW, Lee TS, Hong DG, No JH, Park DC, Bae JM, et al. Practice guidelines for management of uterine corpus cancer in Korea: a Korean Society of Gynecologic Oncology Consensus Statement. J Gynecol Oncol. 2017; 28:e12.

31. Nakano T. Status of Japanese radiation oncology. Radiat Med. 2004; 22:17–9.
32. Teshima T, Owen JB, Hanks GE, Sato S, Tsunemoto H, Inoue T. A comparison of the structure of radiation oncology in the United States and Japan. Int J Radiat Oncol Biol Phys. 1996; 34:235–42.

33. Tanikawa T, Ohba H, Ogasawara K, Okuda Y, Ando Y. Geographical distribution of radiotherapy resources in Japan: investigating the inequitable distribution of human resources by using the Gini coefficient. J Radiat Res. 2012; 53:489–91.

34. Susumu N, Sagae S, Udagawa Y, Niwa K, Kuramoto H, Satoh S, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008; 108:226–33.

Fig. 1.
Trends of patient characteristics. (A) Number of patients and facilities per year. (B) Age distribution per year. Patients aged >60 years significantly increased (p<0.001). (C) Histological subtype distribution per year. Trend test revealed significant increases in the frequency of serous adenocarcinoma, and in the population of both serous and clear cell carcinoma (p<0.001).
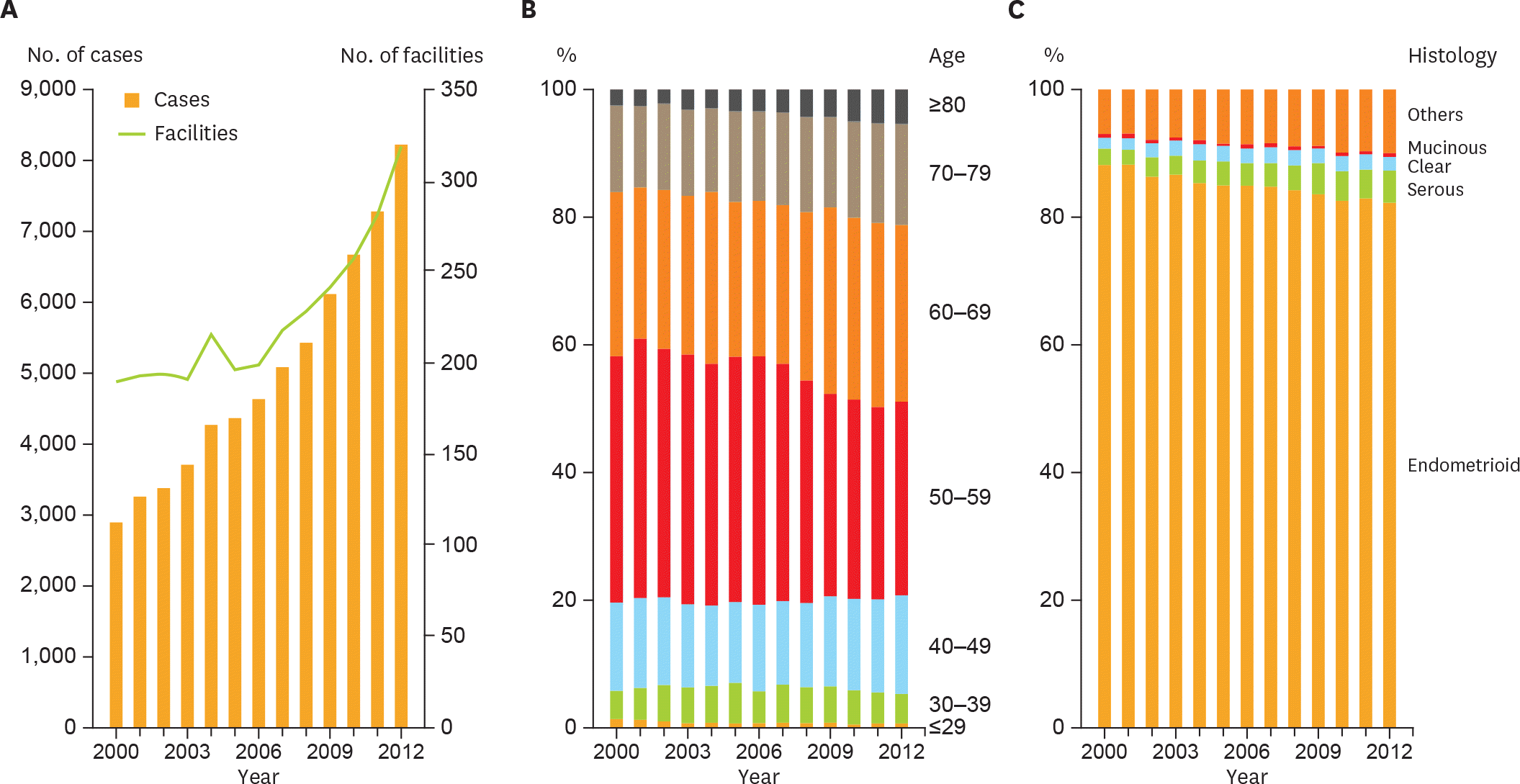
Fig. 2.
Trends of clinical practice in endometrial cancer management. (A) Distribution of initial treatment by year. Among surgeries, the proportion of LA procedures had significantly decreased (Cochran-Armitage trend test, p<0.001). (B) Trend of adjuvant therapy in each year. The proportion of adjuvant chemotherapy had significantly increased among the adjuvant therapies (Cochran-Armitage trend test, p<0.001). EBRT, extra-beam radiation therapy; ICBT, intracavitary brachytherapy; LA, lymphadenectomy.
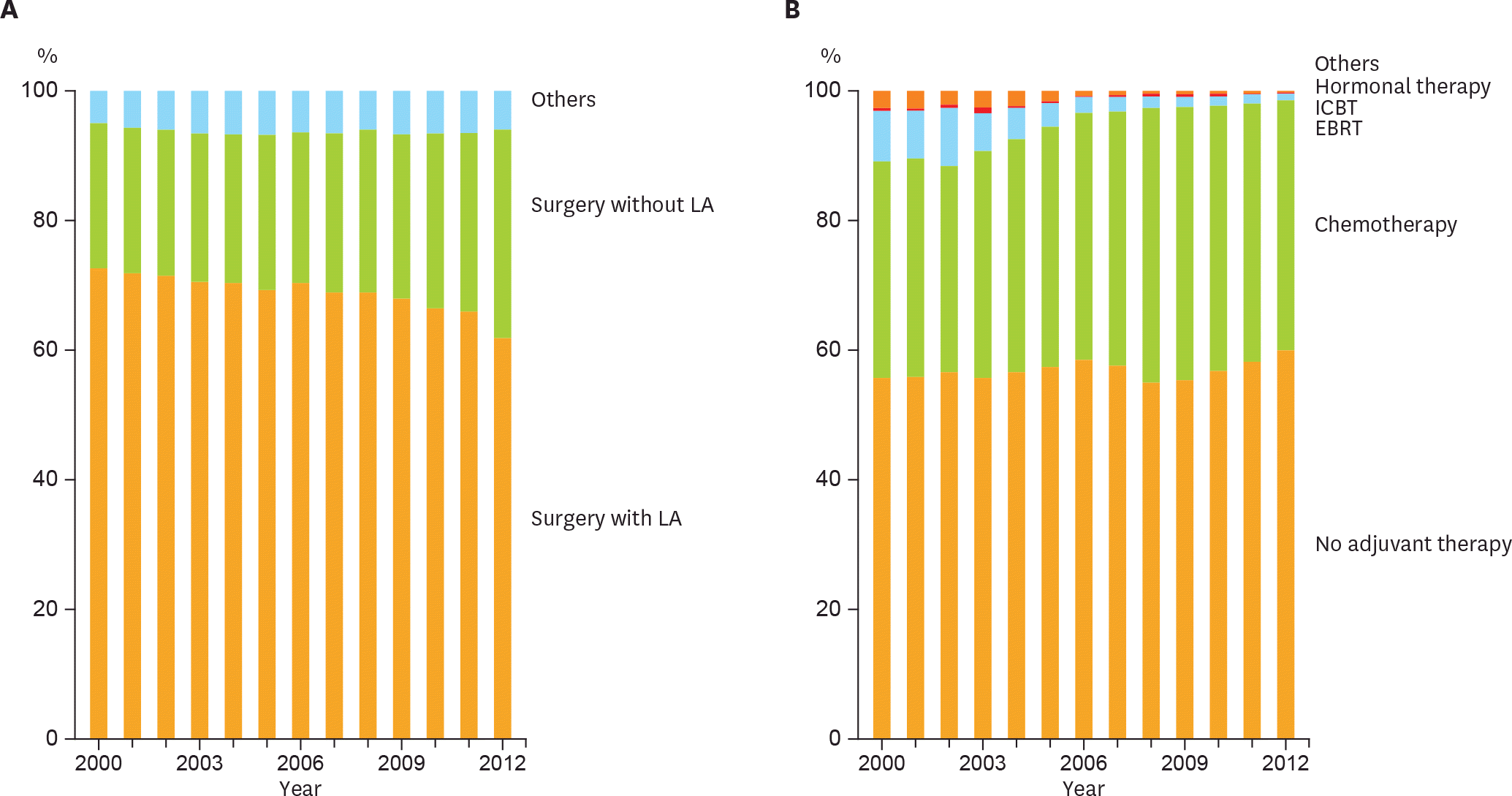
Table 1.
Patient characteristics
EBRT, extra-beam radiation therapy; FIGO, International Federation of Gynecology and Obstetrics; ICBT, intracavitary brachytherapy; LA, lymphadenectomy.* For postoperative staging, 1988 FIGO staging criteria were adopted from 2000 to 2011. FIGO clinical staging criteria were also employed from 2000 to 2011. 2009 FIGO staging criteria were adopted for the all cases registered in 2012.
Table 2.
Frequency of LA between pre- and post-guideline publication
| Stages | No. of patients (%) | |||
|---|---|---|---|---|
| 2000–2006 | 2007–2012 (All) | |||
| With LA | Without LA | With LA | Without LA | |
| All* | 18,777 (75.5) | 6,101 (24.5) | 25,747 (70.8) | 10,636 (29.2) |
| Ia† | 2,801 (61.1) | 1,788 (38.9) | 3,049 (55.9) | 2,406 (44.1) |
| Ib† | 6,731 (78.1) | 1,886 (21.9) | 7,167 (75.1) | 2,373 (24.9) |
| Ic | 2,609 (78.8) | 701 (21.2) | 2,952 (77.0) | 883 (23.0) |
| IIa† | 663 (79.4) | 172 (20.6) | 760 (73.1) | 279 (26.9) |
| IIb | 1,032 (81.9) | 228 (18.1) | 1,193 (79.6) | 305 (20.4) |
| IIIa | 2,180 (79.4) | 564 (20.6) | 2,494 (78.1) | 698 (21.9) |
| IIIb | 73 (66.4) | 37 (33.6) | 67 (60.9) | 43 (39.1) |
| IIIc† | 2,132 (93.8) | 142 (6.2) | 2,280 (92.0) | 197 (8.0) |
| IVa | 45 (48.9) | 47 (51.1) | 41 (45.1) | 50 (54.9) |
| IVb | 460 (50.4) | 453 (49.6) | 584 (46.3) | 676 (53.7) |
Table 3.
Multivariate analysis of the influence of the guideline publication




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download