Abstract
Objective
MicroRNAs (miRNAs) play a vital role in pathogenesis and progression of many cancers, including cervical cancer. However, importance of serum level of miR-101 in cervical cancer has rarely been studied. In the present study, clinical significance and prognostic value of serum miR-101 for cervical cancer was investigated.
Methods
Association between miR-101 level in cervical cancer tissues and prognosis of patients was analyzed by using data retrieved from The Cancer Genome Atlas (TCGA) database, which was followed with our clinical study in which miR-101 serum level comparison between cervical cancer patients and healthy controls was conducted by real-time quantitative polymerase chain reaction (PCR).
Results
TCGA database demonstrated that miR-101 was down-regulated in cervical cancer tissues compared with normal cervical tissues, and univariate Cox regression analysis indicated that decreased miR-101 expression was a highly significant negative risk factor. Similar trend was found in the serum miR-101. Serum level of miR-101 was associated with International Federation of Gynecology and Obstetrics (FIGO) stage (p=0.003), lymph node metastasis (p=0.001), and serum squamous cell carcinoma antigen (SCC-Ag) level >4 (p=0.007). The overall survival time of cervical cancer patients with a higher level of serum miR-101 was significantly longer than that of patients with a lower level of serum miR-101. Moreover, multivariate Cox regression analysis indicated that the down-regulated serum level of miR-101 was an independent predictor for the unfavorable prognosis of cervical cancer.
Go to : 
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108.

2. Vaccarella S, Lortet-Tieulent J, Plummer M, Franceschi S, Bray F. Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur J Cancer. 2013; 49:3262–73.

4. Esquela-Kerscher A, Slack FJ. Oncomirs – microRNAs with a role in cancer. Nat Rev Cancer. 2006; 6:259–69.

5. Weiland M, Gao XH, Zhou L, Mi QS. Small RNAs have a large impact: circulating microRNAs as biomarkers for human diseases. RNA Biol. 2012; 9:850–9.
6. Nugent M, Miller N, Kerin MJ. Circulating miR-34a levels are reduced in colorectal cancer. J Surg Oncol 2012;106:947–52.
7. Schetter AJ, Okayama H, Harris CC. The role of microRNAs in colorectal cancer. Cancer J. 2012; 18:244–52.

8. Lim LP, Glasner ME, Yekta S, Burge CB, Bartel DP. Vertebrate microRNA genes. Science. 2003; 299:1540.

10. Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005; 65:7065–70.

11. Liu L, Guo J, Yu L, Cai J, Gui T, Tang H, et al. miR-101 regulates expression of EZH2 and contributes to progression of and cisplatin resistance in epithelial ovarian cancer. Tumour Biol. 2014; 35:12619–26.

12. Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006; 9:189–98.

13. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015; 43:e47.

14. Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005; 65:6029–33.

15. Tian Y, Luo A, Cai Y, Su Q, Ding F, Chen H, et al. MicroRNA-10b promotes migration and invasion through KLF4 in human esophageal cancer cell lines. J Biol Chem. 2010; 285:7986–94.

16. Calin GA, Cimmino A, Fabbri M, Ferracin M, Wojcik SE, Shimizu M, et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci U S A. 2008; 105:5166–71.

17. Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol. 2009; 16:23–9.

18. Yang Z, Chen S, Luan X, Li Y, Liu M, Li X, et al. MicroRNA-214 is aberrantly expressed in cervical cancers and inhibits the growth of HeLa cells. IUBMB Life. 2009; 61:1075–82.

19. Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008; 18:997–1006.

20. Piao J, You K, Guo Y, Zhang Y, Li Z, Geng L. Substrate stiffness affects epithelial-mesenchymal transition of cervical cancer cells through miR-106b and its target protein DAB2. Int J Oncol. 2017; 50:2033–42.

21. Wang C, Zhou B, Liu M, Liu Y, Gao R. miR-126-5p restoration promotes cell apoptosis in cervical cancer by targeting Bcl2l2. Oncol Res. 2017; 25:463–70.

22. Guo M, Zhao X, Yuan X, Jiang J, Li P. MiR-let-7a inhibits cell proliferation, migration, and invasion by down-regulating PKM2 in cervical cancer. Oncotarget. 2017; 8:28226–36.

23. Liu Z, Wang J, Mao Y, Zou B, Fan X. MicroRNA-101 suppresses migration and invasion via targeting vascular endothelial growth factor-C in hepatocellular carcinoma cells. Oncol Lett. 2016; 11:433–8.

24. Liang X, Liu Y, Zeng L, Yu C, Hu Z, Zhou Q, et al. miR-101 inhibits the G1-to-S phase transition of cervical cancer cells by targeting Fos. Int J Gynecol Cancer. 2014; 24:1165–72.
25. Lin C, Huang F, Shen G, Yiming A. MicroRNA-101 regulates the viability and invasion of cervical cancer cells. Int J Clin Exp Pathol. 2015; 8:10148–55.
26. Oh J, Lee HJ, Lee TS, Kim JH, Koh SB, Choi YS. Clinical value of routine serum squamous cell carcinoma antigen in follow-up of patients with locally advanced cervical cancer treated with radiation or chemoradiation. Obstet Gynecol Sci. 2016; 59:269–78.

27. Esajas MD, Duk JM, de Bruijn HW, Aalders JG, Willemse PH, Sluiter W, et al. Clinical value of routine serum squamous cell carcinoma antigen in follow-up of patients with early-stage cervical cancer. J Clin Oncol. 2001; 19:3960–6.

28. Li J, Chen P, Mao CM, Tang XP, Zhu LR. Evaluation of diagnostic value of four tumor markers in bronchoalveolar lavage fluid of peripheral lung cancer. Asia Pac J Clin Oncol. 2014; 10:141–8.

29. Cao X, Zhang L, Feng GR, Yang J, Wang RY, Li J, et al. Preoperative Cyfra21-1 and SCC-Ag serum titers predict survival in patients with stage II esophageal squamous cell carcinoma. J Transl Med. 2012; 10:197.

30. Torre GC. SCC antigen in malignant and nonmalignant squamous lesions. Tumour Biol. 1998; 19:517–26.

31. Ryu HK, Baek JS, Kang WD, Kim SM. The prognostic value of squamous cell carcinoma antigen for predicting tumor recurrence in cervical squamous cell carcinoma patients. Obstet Gynecol Sci. 2015; 58:368–76.

Go to : 
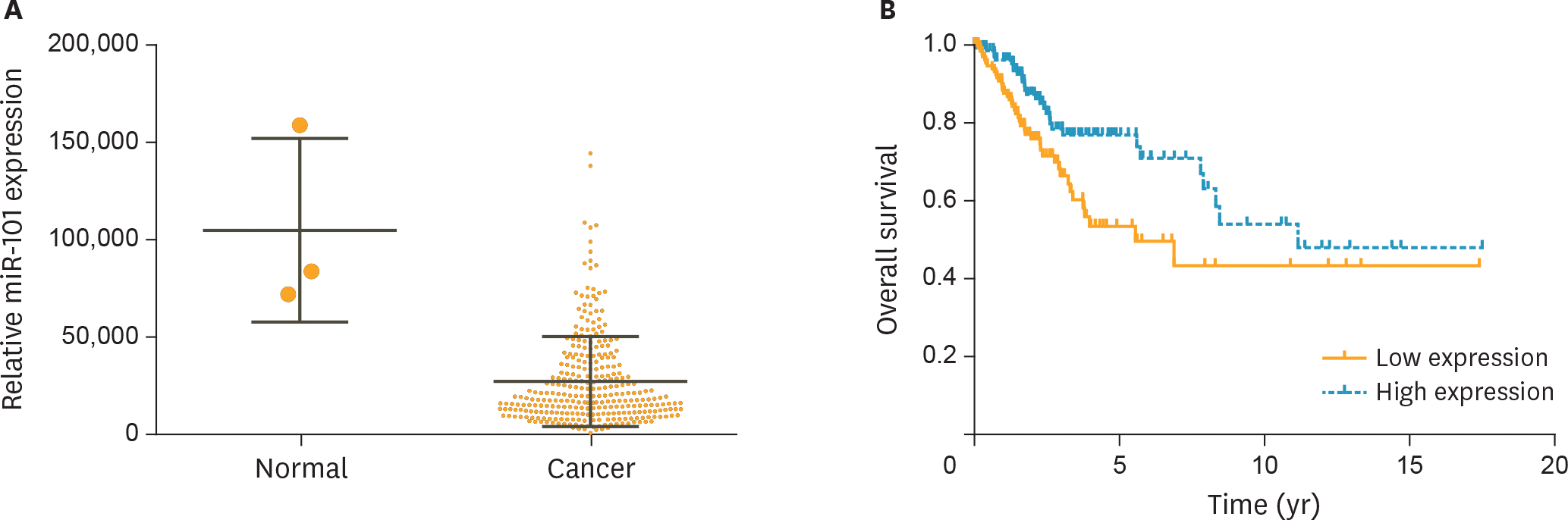 | Fig. 1.Expression level of miR-101 in cervical cancer was significantly decreased and positively associated with the prognosis of patients. (A) MiR-101 expression levels of cervical cancer patients (n=307) and healthy persons (n=3) from the TCGA database were analyzed. (B) The survival analysis for 2 groups of patients with low or high expression levels of miR-101. TCGA, The Cancer Genome Atlas. |
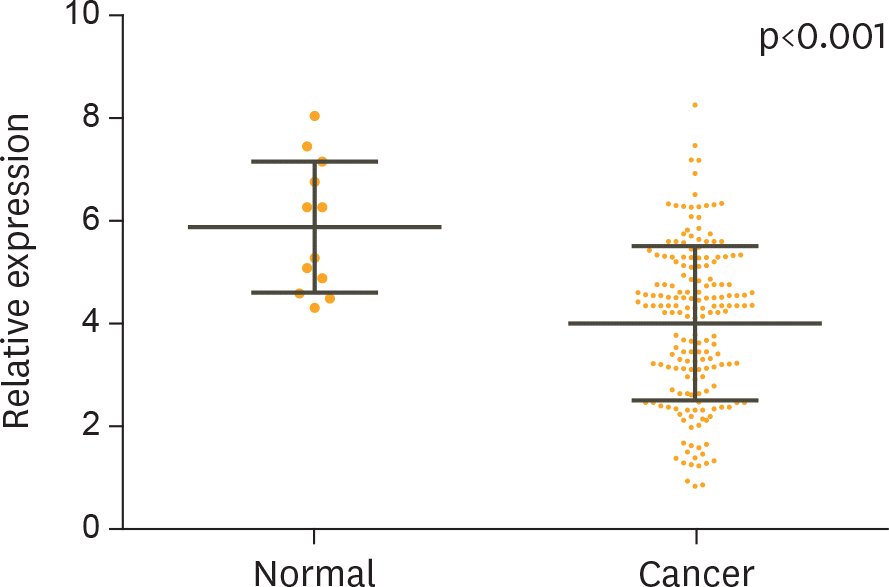 | Fig. 2.The relative expression levels of miR-101 for 182 cervical cancer patients, before and after treatment, and 12 healthy women. The average value is indicated by the horizontal lines among the spots. The serum level of miR-101 was significantly higher in the healthy women compared with that from cervical cancer patients (p<0.001). |
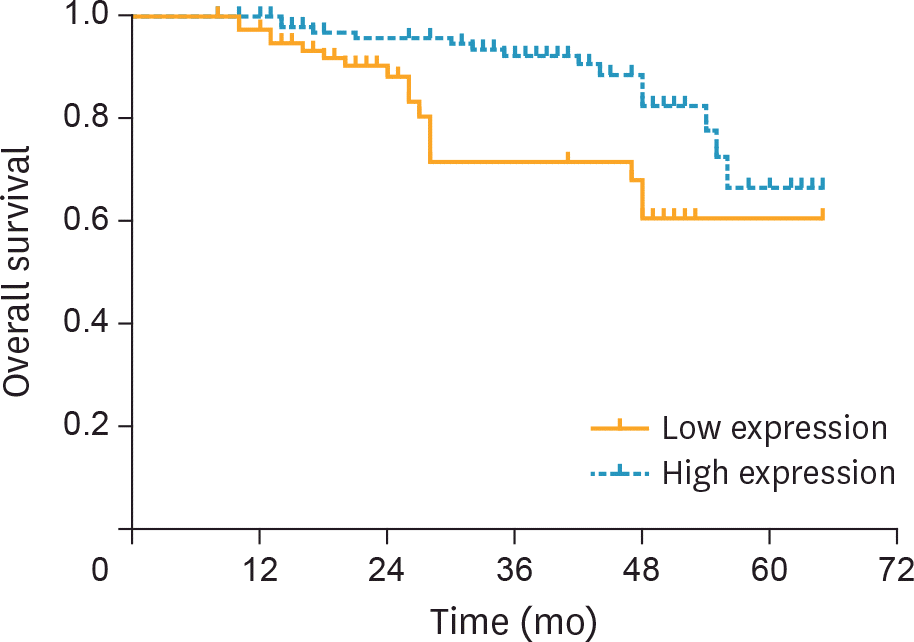 | Fig. 3.The association between serum miR-101 level and overall survival time was analyzed by Kaplan-Meier method. The survival period was shorter in the cervical cancer patients with a lower expression level of miR-101 (p=0.004). |
Table 1.
Correlation of clinicopathological characteristics and serum miR-101 expression in the cervical cancer patients
Table 2.
Univariate and multivariate analyses of prognostic parameters in cervical cancer using the Cox regression model




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download