Abstract
Malignant melanomas of the uterus, either primary or metastatic, are extremely rare. They can be mistaken as other tumors, such as uterine sarcomas during diagnosis. We describe here the first case of a metastatic melanoma of the uterus with peritoneal seeding in a young woman. It was first diagnosed as a uterine sarcoma from a frozen-section biopsy.
Malignant melanoma is an aggressive and metastatic cancer originating from melanocytes. It occurs at various sites, most often in the skin and much less frequently in the choroid layers of the eyes, oral cavity, nasal mucosa, leptomeninges, pharynx, esophagus, bronchus, and vaginal and anorectal mucosa [1]. Common metastatic sites of malignant melanomas are the lung, liver, lymph nodes, brain and meninges, bone, and gastrointestinal tract. Less frequently, they form metastases to the scalp, dura, eye, bile duct, duodenum, uterine cervix, vagina, rectum, anus, and peripheral nerves [2]. However, there is no known case of peritoneal seeding of a malignant melanoma.
We describe here the case of a young woman with pathologically confirmed peritoneal seeding of a malignant melanoma, that was first considered to be a uterine sarcoma.
A 22-year-old nulliparous, woman from Mongolia visited a department of obstetrics and gynecology complaining of lower abdominal pain that had developed about 1 week previously. She had undergone a pelviscopic myomectomy for uterine myomas 2 years before this visit. On transvaginal ultrasonography, an 8cm sized uterine solid mass and other pelvic masses were found, but both ovaries seemed intact. Computed tomography (CT) scanning showed similar findings to the ultrasonography. With the diagnosis of a gynecologic malignancy, she was referred to our hospital.
At initial workup in our hospital, moderate anemia was found: the patient's hemoglobin concentration was 8.7 mg/dL and the hematocrit 28.8%. The tumor marker CA-125 level was elevated (226 U/mL). Re-evaluation of the CT scan showed multiple solid enhancing masses in the uterus, peritoneal cavity, and retroperitoneal cavity with moderate amount of ascites. No lymph node was significantly enlarged (Fig. 1). Intravenous leiomyomatosis with pseudo-Meig syndrome was suspected, but the possibility of a uterine sarcoma could not be excluded.
After treating the patient's anemia with a blood transfusion, explorative laparotomy was carried out. Multiple solid masses were found in the omentum, the mesocolon, the abdominal peritoneum, the right ovary, and as well as in the uterus. Some biopsies of the omental and uterine masses were sent for frozen sectioning. The histopathology diagnosis was of a malignant tumor such as an endometrial stromal sarcoma or a leiomyosarcoma. Then, we performed total abdominal hysterectomy, right salpingo-oophorectomy, left salpingectomy, omentectomy, appendectomy, colon mesentery, and peritoneal mass removal. Moderate amounts of ascites were also aspirated. Because the patient was young, and she and her husband strongly wanted to save her ovary, we preserved the left ovary after explaining the risk of this procedure given her medical condition.
Unlike the presumptive clinical impression or the frozen-section biopsy result, the final histopathology finding with immunochemistry was of a malignant melanoma. The mitotic count was 11 per 10 high power fields and tumor cell necrosis was seen. The tumor was immunopositive for S-100, and HMB-45, and focally positive for cyclin D1, but negative for CD10, smooth muscle actin, and desmin (Fig. 2). The tumor had infiltrated the uterus, right adnexa, omentum, colon mesentery, appendix, and peritoneal mass, but not he uterine cervix or vagina. Ascitic fluid histopathology results the were negative.
After postoperative care, the patent was transferred to the department of hemato-oncology for further evaluation and chemotherapy. Whole body 2-[fluorine-18]-fluoro-2-deoxy-D-glucose positive emission tomography-CT and brain magnetic resonance imaging scans were performed with the aim of determining the primary tumor site, but no abnormal enhancing lesion was found. Except for a small nevus-like lesion in her right lower extremity, there was no pigmented skin lesion including the vulvar area. Because the couple wanted to return to Mongolia, a skin biopsy was not performed, and she was discharged from our hospital.
Malignant melanomas are composed of melanocytes, which are neural crest-derived cells that produce melanin [3,4]. Approximately 3% involve the female genital tract [5]. The majority occur in the vulva and vagina, but rarely in the uterine cervix, ovaries, or uterus [6]. Malignant melanomas of the uterus are extremely rare: only four cases of primary malignant melanomas and 10 cases of metastatic malignant melanomas have been reported. In cases of primary malignant melanomas, all have affected older women and their chief complaints were postmenopausal bleeding [6,7]. Moreover, there has been no known case of peritoneal seeding of a metastatic melanoma.
The prognosis for patients with a malignant melanoma of the uterus is very poor, whether the tumor is primary or metastatic. In previous cases, the longest survival was 1 year. In 2012, Tas [8] reported a median survival of 7 months in patients with melanoma metastases to visceral sites other than the lung, and the numbers of metastatic sites were the most important prognostic factors. The 1-year survival rate for patients with a solitary metastasis was 36%, 13% for patients with two sites, and less than 1% for patients with three or more metastatic sites [6,8].
There is no consensus on treatment of a metastatic melanoma of the uterus, because of its rarity. Systemic therapy is the mainstay of therapy for patients with a distant metastatic melanoma. The single agent dacarbazine is the standard treatment. Combination chemotherapy might yield higher response rate than dacarbazine monotherapy, but this does not increase survival and is associated with higher toxicity. Immunotherapy such as high doses of interleukin-2 or interferon-α-2b can be used to treat metastatic melanomas [9].
Metastatic melanomas can be confused with epithelioid leiomyosarcomas. In the presented case, as elsewhere, the tumor was first diagnosed as a uterine sarcoma based on frozen-section histopathology. Immunochemistry is helpful for the differential diagnosis and positive results for S-100, HMB-45, and negative expression of smooth muscle markers favor the diagnosis of a melanoma [10].
In conclusion, although rare, metastatic malignant melanomas should be considered in the differential diagnosis of a uterine malignancy, especially if the patient has a nevus. This case is presented because, to our knowledge, it is the first case of a malignant melanoma with peritoneal seeding in a young woman.
Figures and Tables
Fig. 1
(A) Enhanced computed tomography scan shows uterine mass (arrow). (B) Ascites, heterogenously enhanced peritoneal seeding and retroperitoneal seeding masses were seen. Lymph node enlargement was not shown.
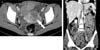
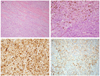
Fig. 2
(A) Microscopic findings of the tumor. Tumor cells show infiltrative growth through myometrium (H&E, ×100). (B) There are focal areas with melanin pigment deposition by the tumor cells (H&E, ×200). Immunohistochemical staining demonstrates that the tumor cells are positive for S-100 (C) (×200) and also positive for HMB-45 (D) (×200).
References
1. Iijima S, Oka K, Sasaki M, Tateishi Y, Saito H, Sandoh N, et al. Primary jejunal malignant melanoma first noticed because of the presence of parotid lymph node metastasis. J Am Acad Dermatol. 2003; 49:319–323.
2. Lee YT. Malignant melanoma: pattern of metastasis. CA Cancer J Clin. 1980; 30:137–142.
3. Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. J Biol Chem. 2007; 282:27557–27561.
4. Bharti K, Nguyen MT, Skuntz S, Bertuzzi S, Arnheiter H. The other pigment cell: specification and development of the pigmented epithelium of the vertebrate eye. Pigment Cell Res. 2006; 19:380–394.
5. Gungor T, Altinkaya SO, Ozat M, Bayramoglu H, Mollamahmutoglu L. Primary malignant melanoma of the female genital tract. Taiwan J Obstet Gynecol. 2009; 48:169–175.
6. Ariel IM. Malignant melanoma of the female genital system: a report of 48 patients and review of the literature. J Surg Oncol. 1981; 16:371–383.
7. Luxman D, Jossiphov J, Cohen JR, Wolf Y, David MP. Uterine metastasis from vulvar malignant melanoma. A case report. J Reprod Med. 1997; 42:244–246.
8. Tas F. Metastatic behavior in melanoma: timing, pattern, survival, and influencing factors. J Oncol. 2012; 2012:647684.
9. Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: an overview. Oncology (Williston Park). 2009; 23:488–496.
10. Toledo G, Oliva E. Smooth muscle tumors of the uterus: a practical approach. Arch Pathol Lab Med. 2008; 132:595–605.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download