Abstract
Neural tube defects are the major targets of prenatal diagnoses, along with Down syndrome. Prenatal diagnosis of spina bifida is possible at second trimester of gestation through α-fetoprotein and acetylcholinesterase biochemistry assays and ultrasound. In particular, the discovery of characteristic intracranial signs on ultrasound leads to a very high diagnosis rate. However, it is rare for spina bifida to present without intracranial signs while also showing normal values of maternal serum α-fetoprotein, amniotic fluid α-fetoprotein, and acetylcholinesterase. In our hospital, a fetus with spina bifida was delivered at 37+5 weeks' gestation by cesarean section, and was continually followed up over 2 years to date.
Spina bifida refers to neural tube defects (NTDs) at the spine. NTDs are generally classified as either closed or open defects. Closed NTDs, in which the nerve tissue is covered by skin and not exposed outside the body, are more common than open defects; the symptoms are minor or nonexistent. In contrast, open NTDs involve neural tube exposure through spinal cord defects, and present as a meningocele sac or a ruptured meningocele. In the case of open NTDs, Chiari type II anomaly develops when intracranial brain structures herniate toward the spinal canal as cerebrospinal fluid escapes through the NTD area. Such malformations present as characteristic intracranial signs on ultrasound, such as ventriculomegaly, small biparietal diameter, scalloping of the frontal bone ("lemon" sign), obliteration of the cisterna magna, and compressed cerebellum ("banana" sign) [1]. The major distinction between these 2 groups is the presense or absence of skin covering the mass. Most of the other findings result from this difference, i.e., increased α-fetoprotein levels (AFP), Chiari II malformation, and hydrocephalus in open NTD, and normal AFP levels, absence of Chiari II, and normal ventricles in closed NTD [2].
Patients with spina bifida may have lower-extremity weakness or paralysis, some potential orthopedic problems (i.e., club foot, pelvic dislocation, and scoliosis), inadequate bowel or bladder control and developmental impairment. Thus, pregnancy termination could be recommended as an option when a prenatal diagnosis is made after appropriate counseling was provided.
A 25-year-old woman, gravida 1 para 0 abortus 1, who underwent regular prenatal checkups, presented to our hospital at 22+6 weeks' gestation with suspected spina bifida. She had a regular 28-day menstrual cycle, with moderate menstrual flow for 4 to 5 days. Her last menstrual period was on January 1, 2010, and the expected date of confinement was November 6, 2010. The patient's medical history was unremarkable. In particular, there was no notable history of genetic disease or delivery of a baby with congenital birth defects among her close relatives or those of her spouse. The results of the quad screen test performed at 16+4 weeks' gestation at a private clinic showed that maternal serum AFP was at 1.8 multiples of the median (MoM), within the normal range (2.5 MoM).
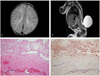
Abdominal ultrasound showed that the fetus was in a cephalic presentation with a biparietal diameter of 5.4 cm (22+5 weeks), abdominal circumference 17.7 cm (22+2 weeks), and femur length 3.8 cm (21+5 weeks), corresponding to 22+2 weeks' gestation. No intracranial signs were observed; however, a hypodense cystic mass (2.1'1.7 cm in diameter) was found in the lumbosacral area (Fig. 1A, B). No other concomitant malformations were found. We performed amniocentesis at 22+6 weeks' gestation. The amniotic fluid AFP level was found to be within normal range, and native-polyacrylamide gel electrophoresis test of AChE results as well as the karyotype were normal (Fig. 1C). Termination was recommended with appropriate counseling, but the patient declined; thus, outpatient follow-up was conducted. The lumbar cyst increased in size, measuring 3.2'1.8 cm at 26 weeks' gestation and 3.5'2.5 cm at 28 weeks' gestation; at 35 weeks' gestation, the size increased to 6.6'3.9 cm. On October 21, 2010, the fetus was delivered by an elective cesarean section at 37+5 weeks' gestation. The fetus was female, 3.46 kg in weight, and had an Apgar score of 7 to 9 points. Grossly, the sac was present measuring up to 7.7 cm in diameter. The lower limb motor power and bowel continence were normal. The infant passed urine normally. Anocutaneous reflex was present. The systemic examination was essentially normal. Echocardiogram and ultrasound of the brain, kidneys, ureters, and bladder were within normal limits. The karyotype study performed after birth showed 46,XX. Moreover, no clearly abnormal findings were seen on brain magnetic resonance imaging (MRI) performed immediately after birth (Fig. 2A). Spinal MRI showed that the compromised level was L3-4, with a suspicion of a meningocele (Fig. 2B). Meningocele repair was performed on day 6 after birth and was uneventful. The histopathologic examination revealed a meningocele diagnosis (Fig. 2C, D). There was no epidermis, and thus, no skin-covered meningocele. At present, the child is 34 months old and being followed by the pediatrics and neurosurgical departments of our hospital, without any additional procedures or surgeries. No particular problems exist.
Spina bifida arises from a deficit in primary neurulation on days 25 to 28 of pregnancy, in which the caudal region of the neural tube (neuropore) fails to fuse [3]. The incidence varies among regions and ethnicities. In the United States, the incidence has not decreased below 3.4 cases per 10,000 live births despite folic acid fortification [4]. Although the causative factors are not clearly established, spina bifida is thought to be a complex multifactorial disease influenced by interactions between related genetic factors, diet, diabetes mellitus, obesity, hyperthermia, certain medications including antiepileptic drugs, and smoking [5]. An identified genetic factor is 5,10-methylene tetrahydrofolate reductase polymorphism, which is both directly and indirectly involved in folic acid and homocysteine metabolism [5]. The patient had no medication use during pregnancy, no concomitant diabetes, and no relevant family history; however, correlations with genetic and environmental factors were not investigated.
In 2003, the American College of Obstetricians and Gynecologists recommended that maternal serum AFP be measured in all pregnant women as an NTD screening test [6]. Maternal serum AFP testing is most accurate at 16 to 18 weeks' gestation, but is usually conducted between weeks 15 and 22. The age, weight, ethnicity, diabetes history, number of fetuses, and gestational weeks also be considered. However, this is only a screening test, limited by its high false-positive rate and low sensitivity, and does not have significant weight in terms of diagnosis; thus, genetic counseling and diagnostic examinations must also be conducted.
Previously, the gold standard for the prenatal diagnosis of NTD was increased amniotic fluid AFP and AChE levels. When neural tissue is exposed to the amniotic fluid, amniotic fluid AChE increases; when both of these markers are measured, the detection rate of anencephaly and open spina bifida is 100% [7]. False-negative values are extremely rare for amniotic fluid AChE in terms of NTD detection [8]. Additionally, the fetal karyotype can be examined to rule out chromosomal abnormalities. When the maternal age was set as the standard, chromosomal abnormalities were found in 0.3% of the normal pregnancy group, whereas chromosomal abnormalities were found in 16.3% of NTD fetuses [9]. However, it was also reported that when maternal serum AFP alone was elevated and ultrasound findings were normal, chromosomal abnormalities were discovered in only 0.61% of cases [10]. In the second trimester of pregnancy, the postprocedure pregnancy loss of amniocentesis reaches 1.4% [11]. The improved resolution of ultrasound has reportedly improved the accuracy of NTD diagnosis from ultrasound over amniocentesis in patients with elevated maternal serum AFP levels. The possibility of an anomaly is low if the ultrasound is normal; reports have proposed that subsequent amniocentesis must be decided by the patient, guided by sufficient information [12].
The fetal spine is sufficiently ossified at approximately 16 weeks' gestation, and is readily observable through ultrasound. At this time, splaying of the posterior ossification centers, a meningocele or myelomeningocele sac, the presence of placode on the sac surface, or neural elements bridging across the sac are direct evidences of spina bifida. However, observation can be difficult if the mother is obese, if the fetus is in the persistent spine posterior position, or if the sac has ruptured. Rather than these direct signs, indirect secondary signs of the fetal skull and brain may be easier to observe in the early stages. It has been reported that 99.6% of NTD fetuses present with at least one intracranial sign [1]. The size of the NTD lesion is reportedly associated with intracranial sign manifestation [13]; however, another report has found no association [10]. The false-positive rate of intracranial sign exhibited by normal fetuses without spina bifida is known to be approximately 1% [1].
Although intracranial signs are critical for open spina bifida diagnosis during ultrasound, they could be absent. Thus, the sagittal images of the spine must be carefully observed for the presence of a meningocele sac. In the present case, diagnosis was delayed in a private clinic because no abnormal findings were detected during the prenatal screening, including the absence of clear intracranial signs and the normal quad screen test, and the spinal mass was initially overlooked. In addition, intracranial signs were believed to be absent in the present case because the fetus had a meningeal sac, which prevented the free leakage of cerebrospinal fluid into the amniotic cavity, thus reducing the probability of intracranial sign manifestation.
There is no conclusive evidence showing cesarean section improves outcome in children with spina bifida. However, cesarean section to decrease the risk of disruption of large lesions (>6 cm) might be justified [14]. In the present case, the lesion was already 6.6 cm at 35 weeks' gestation. We suggested delivery by elective cesarean section, which performed at 37+5 weeks' gestation upon our clinical decision.
Figures and Tables
Fig. 1
(A,B) At 22+6 weeks' gestation, sonography shows normal range of cistern magna, no intracranial signs at axial view. Cystic mass is present in the lumbosacral area. (C) Native-polyacrylamide gel eletrophoresis analysis of amniotic fluid acetylcholinesterase was performed at 22+6 weeks' gestation. 1→ slow migrating thin band with specific acetylcholinesterase inhibitor; a negative control (lane NC). 2→ additional fast moving thick band of specific acetylcholinesterase; a positive control (lane PC). Lane Pt, patient shows a thin negative pattern.
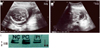
Fig. 2
(A) Immediate postnatal brain magnetic resonance imaging (MRI) shows normal findings. There is no lemon sign and no ventriculomegaly. (B) Spinal MRI. Sagittal T2-weighted MRI image of the lumbar meningocele. The level of defect was between L3 and L4. (C) The histopathologic examination revealed a meningocele (H&E, ×100). (D) Strong reactivity to epithelial membrane antigen (EMA) was seen in the epithelial lining and granules on the surface of the arachnoid and pia mater (EMA, ×100).
References
1. Nicolaides KH, Campbell S, Gabbe SG, Guidetti R. Ultrasound screening for spina bifida: cranial and cerebellar signs. Lancet. 1986; 2:72–74.
2. Pierre-Kahn A, Sonigo P. Lumbosacral lipomas: in utero diagnosis and prognosis. Childs Nerv Syst. 2003; 19:551–554.
3. Nievelstein RA, Hartwig NG, Vermeij-Keers C, Valk J. Embryonic development of the mammalian caudal neural tube. Teratology. 1993; 48:21–31.
4. Boulet SL, Yang Q, Mai C, Kirby RS, Collins JS, Robbins JM, et al. Trends in the postfortification prevalence of spina bifida and anencephaly in the United States. Birth Defects Res A Clin Mol Teratol. 2008; 82:527–532.
5. Kondo A, Kamihira O, Ozawa H. Neural tube defects: prevalence, etiology and prevention. Int J Urol. 2009; 16:49–57.
6. Sebire NJ, Spencer K, Noble PL, Hughes K, Nicolaides KH. Maternal serum alpha-fetoprotein in fetal neural tube and abdominal wall defects at 10 to 14 weeks of gestation. Br J Obstet Gynaecol. 1997; 104:849–851.
7. Loft AG, Hogdall E, Larsen SO, Norgaard-Pedersen B. A comparison of amniotic fluid alpha-fetoprotein and acetylcholinesterase in the prenatal diagnosis of open neural tube defects and anterior abdominal wall defects. Prenat Diagn. 1993; 13:93–109.
8. Boogert A, Aarnoudse JG, De Bruijn HW, Gouw A. False-negative amniotic fluid acetylcholinesterase in a case of meningo-encephalocele. Prenat Diagn. 1989; 9:133–138.
9. Harmon JP, Hiett AK, Palmer CG, Golichowski AM. Prenatal ultrasound detection of isolated neural tube defects: is cytogenetic evaluation warranted? Obstet Gynecol. 1995; 86(4 Pt 1):595–599.
10. Watson WJ, Chescheir NC, Katz VL, Seeds JW. The role of ultrasound in evaluation of patients with elevated maternal serum alpha-fetoprotein: a review. Obstet Gynecol. 1991; 78:123–128.
11. Tabor A, Vestergaard CH, Lidegaard O. Fetal loss rate after chorionic villus sampling and amniocentesis: an 11-year national registry study. Ultrasound Obstet Gynecol. 2009; 34:19–24.
12. Cheschier N. ACOG Committee on Practice Bulletins-Obstetrics. ACOG practice bulletin: Neural tube defects. Number 44, July 2003 (Replaces committee opinion number 252, March 2001). Int J Gynaecol Obstet. 2003; 83:123–133.
13. Bell JE, Gordon A, Maloney AF. The association of hydrocephalus and Arnold: Chiari malformation with spina bifida in the fetus. Neuropathol Appl Neurobiol. 1980; 6:29–39.
14. Anteby EY, Yagel S. Route of delivery of fetuses with structural anomalies. Eur J Obstet Gynecol Reprod Biol. 2003; 106:5–9.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download