Abstract
Objective
To investigate whether natural killer (NK) cell and autoimmune antibody acts synergistically, by the action of autoantibodies to increase NK cell number and cytotoxicity, to decrease uterine blood flow during early pregnancy in pregnant women with a history of recurrent spontaneous abortion (RSA).
Methods
Seventy-five pregnant women (between 5 and 7 weeks gestation) with a history of unexplained RSA were included in the study group. Forty-one pregnant women without a history of RSA were included as controls. All women with a history of RSA were tested for autoantibodies and number of peripheral blood natural killer (pbNK) cell by flow cytometry. Study populations were stratified into four groups by existence of autoantibody and degree of increase of pbNK cells. The uterine radial artery resistance index (RI) was measured by color-pulsed Doppler transvaginal ultrasound.
Results
The mean RI of the autoimmune antibody-positive (AA+) group (0.63±0.09) was significantly higher than that of the normal control group (0.53±0.10, P=0.001). The mean RI of the AA+/only-NK elevated (eNK) group (0.63±0.09) was significantly higher than those of the only-AA+ group (0.55±0.07, P=0.019) and the only-eNK group (0.57±0.07, P=0.021).
Conclusion
Concurrent elevation in NK cells and autoimmunity results in decreased uterine blood flow during early pregnancy. However, the majority of cases of RSA remain unexplained and larger scale studies are needed to confirm our conclusion and to develop diagnostic and therapeutic plans for women with a history of RSA.
Recurrent spontaneous abortion (RSA) has been defined as three or more pregnancy losses before 20 weeks from the last menstrual period, though, some investigators include two or more miscarriages in their series [1]. RSA occurs in about 1% to 2% of all pregnancies [2]. The etiologic origins of RSA have been proposed as genetic, anatomic, endocrine, infectious, immunologic, or thrombotic, among others. In addition, a significant proportion of RSA cases, more than a half, remain unexplained and unresolved despite broad investigations [3,4].
Uterine natural killer (uNK) cells in the endometrium are thought to support remodeling of the uterine spiral arteries and to facilitate successful placentation through the regulation of trophoblast invasion [5]. An abnormal increase in the peripheral blood natural killer (pbNK) cell fraction is associated with RSA and infertility. Furthermore, downregulation of the natural killer (NK) cells is reportedly associated with a favorable pregnancy outcome [6]. However, the exact pathogenic mechanism behind the role of NK cells in human reproduction is unclear. Pathogenic autoantibodies such as antiphospholipid antibodies (APAs), antithyroid antibodies (ATAs), and other autoimmune antibodies have been reported to induce not only impaired blood circulation at the maternal-fetal interface, but also an inflammatory immune response which is related to RSA [7,8]. Moreover, women with RSA had significantly more positive test results for one or both thyroid antibodies (peroxidase and thyroglobulin) than fertile controls [9,10].
Uterine hemodynamic changes in early pregnancy seem to be important factors in determining pregnancy outcomes. To study these changes, Doppler ultrasound has been used to assess blood flow impedance. The blood supply to the uterus is high in the late luteal phase at the time of the implantation of the blastocyst into the endometrium [11]. Some studies have reported that uterine artery Doppler wave forms, characterized by an increased pulsatility index, are indicative of impaired uterine blood flow and are frequently observed with adverse obstetrics outcomes [12]. One reported that women with RSA had a significantly higher uterine artery resistance index (RI) than fertile controls [13]. Another recent study reported that the uterine radial artery more accurately reflects the blood supply to the fetus than the uterine artery in early pregnancy [14].
In this study we designed to investigate whether the uterine blood flow pattern showed distinct pattern by their existence of autoantibody and degree of increase of pbNK cells and whether NK cell and autoimmune antibody acts synergistically decrease in uterine blood flow in women with a history of RSA. We also evaluated the efficacy of low molecular weight heparin (LMWH) treatment in patients in early pregnancy with a history of unexplained RSA who have reduced uterine blood flow.
The study was designed from August 2010 to December 2011. A total of 114 women at 5 to 7 weeks of gestation were enrolled in this study after informed consent was obtained. The study was approved by the institutional review board of Cheil General Hospital and Women's Healthcare Center, Kwandong University College of Medicine.
Subjects were divided into 2 groups: women without a history of repeated pregnancy loss (control group, n=41) who were confirmed to have subsequent normal delivery without any therapy, and women with a history of 2 or more sequential spontaneous pregnancy losses (RSA group, n=73). Unexplained RSA was defined as 2 or more consecutive spontaneous abortions with negative screening for routine RSA evaluations, such as uterine anomalies, parental chromosomal abnormalities, autoimmune diseases including antiphospholipid antibody syndrome (APS) and genital infection (Chlamydia trachomatis, ureaplasma, and mycoplasma). It was confirmed that all control subjects subsequently had a normal delivery without any therapy.
The entire study population was tested for the presence of autoantibodies, such as lupus anticoagulant (LA), anticardiolipin antibody (ACA), and ATA in their blood. In the same blood sample, peripheral blood CD3-/CD56+/CD16+ NK cell fractions among peripheral blood monocytes (PBMC) were checked by flow cytometry. RSA patients were split into two groups according to their autoimmune antibody test results. The autoimmune antibody-positive (AA+) group was defined as those with one or more autoimmune antibodies and the autoimmune antibody-negative (AA-) group was defined as those with no autoimmune antibodies. In addition, each autoimmune antibody group was divided into two groups by their NK cell fractions above or below 12.1%. The cut-off level of 12.1% was determined by our previous study, which was aimed to calculate the relative risk of repeated abortion in patients with unexplained RSA patients with increased pbNK cell [15]. In cases where the NK cell fraction was elevated above 12.1%, subjects were stratified into an elevated NK (eNK) cell group; otherwise, in cases where the NK cell fraction was below 12.1%, they were stratified into the normal NK (nNK) cell group. We then subgrouped RSA patients into 4 groups; AA-/nNK, AA-/eNK, AA+/nNK, and AA+/eNK (Fig. 1).
Uterine color-pulsed Doppler transvaginal ultrasound was performed for evaluation of the uterine radial artery (uRA)-RI on the same day as blood sampling. The uRA-RI was compared between the study and control groups. The mean change in the uRA-RI before and after low molecular weight heparin (LMWH) administration was calculated. Finally we compared the ratio of pregnancies sustained to at least 12 gestational weeks between the normal control and treated RSA groups.
PBMC were isolated from heparinized venous blood samples using Ficoll-Hypaque (Amersham Biosciences, Piscataway, NJ, USA) density centrifugation and washed two times with phosphate-buffered saline (PBS). One hundred microliter of PBMCs were washed in PBS with 1% heat-inactivated fetal bovine serum and 0.09% w/v sodium azide (staining buffer) twice, followed by staining with the fluorochrome-conjugated monoclonal antibodies specific for cell surface antigens. Anti-CD3-FITC, CD4-PE, CD8-PE and CD16/CE56-PE (mouse monoclonal) (Beckman Coulter, Fullerton, CA, USA) were used to detect peripheral blood immune cells. Appropriate isotype controls were used for each antibody. The cell pellets were then fixed and permeabilized for 20 minutes using 250 µL of Cytofix/Cytoperm solution (Pharmingen, San Diego, CA, USA). Immunofluorescence and two-color flow cytometric analysis were performed using a FACS caliber flow cytometer (Becton Dickinson, San Jose, CA, USA) with computer interfacing with BD cell QuestPro for full list-mode data storage, recovery, and analysis. The gate was set on the lymphocyte region using characteristic forward and side scatter parameters. For each sample, 5×106 PBMCs were evaluated and at least 10,000 cells were analyzed.
Uterine blood flow was assessed as the RI of each uterine radial artery, which is the most terminal branch of the uterine feeding vessels. The uterine radial artery was detected at the junction of the uterine endometrium and myometrium using transvaginal sonography at a gestational age of 5 to 7 weeks. All Doppler flow studies and sonograms were read by one person who did not know the subjects' abortion history. The RI was calculated as RI=1-diastolic flow/systolic flow. To reduce errors, the average of the resistance indices of three different sites was calculated.
In RSA patients with an elevated uRA-RI above 0.5, a dose of 40 to 80 mg of LMWH was injected subcutaneously once daily according to body weight. One week after initiating LMWH, the uRA-RI was calculated again for dose adjustment.
We compared the mean uRA-RI of each groups and evaluated the efficacy of LMWH-treatment in patients with a history of unexplained RSA and decreased uterine blood flow. We then compared the ratio of pregnancy sustained to at least 12 gestational weeks between the treated RSA groups and the normal control group. Women with diagnosed abortion with an abnormal chromosomal study or no chromosomal study and loss to follow-up were excluded.
Statistical analysis was performed using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA). Comparison of the mean uRA-RI between groups was performed using one-way ANOVA and Student's t-test. To evaluate the efficacy of LMWH treatment, the differences in RI before and after treatment were calculated using the paired t-test. Moreover, the ratio of pregnancy sustained longer than 12 weeks of gestation was calculated using the chi-square test. Statistical tests were considered significant when the P-value was <0.05.
The mean ages were similar in all groups. Both gravidity and the number of spontaneous abortions were not significantly different between the RSA subgroups. The mean number of spontaneous abortions was 2.5±1.1 (AA-/nNK), 2.3±0.6 (AA-/eNK), 2.0±0.0 (AA+/nNK) and 2.2±0.4 (AA+/eNK) (Table 1).
A total of 37 (50.7%) women with RSA had at least one autoantibody (AA+) and 47 (64.4%) had elevated NK cells (>12%, eNK). A total of 21 (28.8%) women had NK cell elevation only (AA-/eNK) and 26 (35.6%) had both NK cell elevation and autoantibodies (AA+/eNK).
In the AA+ group, 32.9% (24 of 73) had LA and 14 had LA only. Three patients had LA and anticardiolipin IgG (ACAG) and 2 patients had LA, ACAG, and anticardiolipin IgM (ACAM). In 73 subjects in the RSA group, 23.3% (17/73) had ATAs. Nine of them had only ATAs and 7 had LA and ATAs. One patient had only ACAM.
The mean of uRA-RI in the AA+ group was higher than that of normal controls (0.61±0.09 vs. 0.53±0.10, P=0.002). The mean uRA-RI in the AA-group was not significantly higher than that of normal controls (0.58±0.08 vs. 0.53±0.10, P>0.05) (Fig. 2).
The mean uRA-RI between groups was stratified by the autoimmune antibody status and NK cell level. The mean uRA-RI of the AA+/eNK group was significantly higher than that of the only AA+ and only eNK groups (0.63±0.09 vs. 0.55±0.07 vs. 0.57±0.07, P=0.019, P=0.021) (Fig. 3).
In the AA+/eNK group, all 26 patients showed an uRA-RI elevated above 0.5. Seven out of 26 patients had not received LMWH treatment and excluded at obstetric outcome analysis. Among them, 2 patients were revealed to get multiple pregnancies and the uRA-RI was not checked at the second visit. One patient refused to receive LMWH treatment and the pregnancy was terminated at 7 weeks and 6 days of gestation and the chromosomal study turned out to be normal. Another 4 patients, who checked uRA-RI at 5weeks of gestation, were lost to follow up and excluded at obstetric outcome analysis. Nineteen patients of the AA+/eNK group had received treatment with LMWH. One week after treatment with LMWH, the uRA-RI had decreased significantly compared to that the uRA-RI from before LMWH treatment (0.64±0.10 vs. 0.53±0.09, P=0.002) (Fig. 4).
We compared the ratio of pregnancy sustained to at least 12 gestational weeks between the LMWH treatment and control groups. In the LMWH treatment group, 15 out of 17 (88.2%) women sustained at least 12 weeks of gestation, which was similar to the control group in which 35 out of 39 (89.7%) women sustained their pregnancy beyond 12 weeks of gestation. There was no statistically significant difference among the groups (Table 2).
The association between RSA and immunologic phenomena in human reproduction has been studied for many years. However, most of these studies have considered only one aspect of the relationship, that is, RSA and the autoimmune response or RSA and the cellular immune response. This study aimed at evaluating the synergistic role of cellular and autoimmunity, represented by autoimmune antibodies (LA, ACAs, and ATAs) and NK cells in human reproduction, particularly in RSA. This was accomplished by measurement of uterine blood flow.
There have been many reports about the association between the autoimmune response and RSA, especially APS and RSA. This has led to the inclusion of RSA as one of the clinical diagnostic criteria for APS [16]. The antibodies may damage the trophoblasts independent of any presence of thrombosis. Some studies also suggest that APA is associated with increased peripheral blood NK cell cytotoxicity. The numbers and proportions of NK cells are significantly higher in patients with RSA with APS than in APS without RSA [17].
Thyroid autoimmune antibody is also related to impaired cellular and humoral immune responses in women with RSA [18]. Thyroid autoimmune antibody may induce T-cell dysfunction, increased NK cell mass and hyperactivity, resulting in RSA.
The results of this study suggest that autoimmunity plays a pathologic role in abortive events in patients with RSA through a reduction in uterine blood supply that results from inflammation, thrombosis, and infarction [8].
APAs and ATAs augment NK cell numbers and cytotoxicity with resulting recruitment of decidual NK cells. In addition, ATAs induce T-cell dysfunction and stimulate T-cells to secrete IL-2 [18]. Under these conditions, non-cytotoxic decidual NK cells change into cytotoxic CD56+/16+ NK cells. These cells in turn act in several ways, such as mediating trophoblastic cell apoptosis and influencing pro-inflammatory immune cells to cause decidual micro-vessel thrombosis. By these theories, additive or synergistic action between NK cells and autoimmune antibodies causing uterine microvessel thrombosis and RSA would be expected (Fig. 5). But more research is needed for clarification of this relationship.
A number of studies show that poor uterine perfusion might be one of the causes of unexplained infertility [19,20]. In other words, uterine perfusion is known to be an important component in achieving and maintaining a normal pregnancy. The blood supply is regulated by a balance between coagulation and anticoagulation factors and between fibrinolysis and antifibrinolysis [21].
Until now, many studies have shown that uterine artery blood flow is decreased in RSA patients [13,22]. However, measurement of uterine artery blood flow by Doppler ultrasonography to predict pregnancy outcomes is still a subject of much debate. In some studies, measurement of the RI in the first trimester does not predict obstetric outcomes in RSA patients [23]. On the other hand, some studies insist that the uterine blood vessel RI is lower in pregnant women with a live birth than in those who suffer from RSA [24]. Studies concerning uterine blood flow in patients with RSA were poorly performed.
In the present study, we evaluated whether increased NK cell fractions can induce a decrease in uterine blood flow. Our results demonstrated that the mean value of the uRA-RI in RSA patients was increased compared to that of normal controls and especially in RSA patients with elevated NK cells. Those results suggest that decreased uterine blood flow in early pregnancy may be the cause of RSA. Because successful implantation depends on a strong blood supply to the endometrium, an adequate blood supply to the uterus is an essential factor for normal implantation [8].
According to these results, we concluded that peripheral blood NK cells could be used as a marker for uterine microvessel inflammation and thrombosis. There are several studies that support our results. Among them, one study reported strong evidence that elevated numbers of NK cells and increased infiltration of endometrial NK cells are related to pregnancy complications such as miscarriages [25].
Results assessing the treatment effects of LMWH in RSA patients are still conflicting. Some papers insist that LMWH therapy in patients with RSA is not effective because patients with RSA who also have thrombosis are a minority [26]. Other studies have reported that improvement in uterine blood flow by the use of low-dose aspirin and/or LMWH may ensure favorable outcomes in patients with a history of poor obstetric outcomes, especially those with decreased uterine blood flow [27,28].
The increase in uRA-RI in patients with RSA was effectively blunted by LMWH in early pregnancy and there was no significant difference in pregnancy outcomes between the unexplained RSA group and the control group after LMWH treatment. This suggests that LMWH treatment may be effective in ensuring favorable pregnancy outcomes in patients with a history of unexplained spontaneous abortion with elevated uRA-RI through increased uterine blood flow. In this study, LMWH was administered only to patients with an elevated uRA-RI. This treatment improved the uterine blood flow, possibly leading to the more favorable pregnancy outcomes seen in this study.
We propose that the evaluation of pbNK cell fractions and measurement of uterine blood flow using transvaginal Doppler ultrasound are useful ways to predict the risk of recurrent miscarriage and to measure the effects of treatment, which could be immunosuppressant therapy to prevent recurrent miscarriage and anticoagulant therapy to increase uterine blood flow. We also suggest that the results of our study can be used as a prognostic marker of good obstetric outcomes.
In present study, we tried to investigate relationships among uterine blood flow, NK cell and autoimmunity in patients with RSA. In our knowledge, this is the first trial to investigation of novel aspect in immune cause RSA. However, our study was limited by a small study population, the absence of a pathophysiologic investigation of NK cells, and no data regarding cytokines. Larger scale studies with a bio-molecular approach are needed to further substantiate our results.
Figures and Tables
Fig. 1
Demographics of study population; autoimmune antibody-negative (AA-)/normal natural killer (nNK), AA-/elevated natural killer (eNK), autoimmune antibody-positive (AA+)/nNK, and AA+/eNK. RSA, recurrent spontaneous abortion; LA, lupus anticoagulant; pbNK, peripheral blood natural killer.
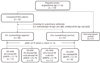
Fig. 2
Comparison of the mean uterine radial artery resistance index among autoimmune antibody-positive (AA+), autoimmune antibody-negative (AA-), and control groups. One-way ANOVA. Values are mean±SD.

Fig. 3
Comparison of the mean uterine radial artery resistance index among the control, autoimmune antibody-negative (AA-)/normal natural killer (nNK), AA-/elevated natural killer (eNK), autoimmune antibody-positive (AA+)/nNK, and AA+/eNK groups. One-way ANOVA. Values are mean±SD.

Fig. 4
Comparison of the mean uterine radial artery resistance index between before treatment with low molecular weight heparin (LMWH) and after 2 weeks of treatment with LMWH. Paired t-test. Values are mean±SD. AA-, autoimmune antibody-negative; nNK, normal natural killer; eNK, elevated natural killer; AA+, autoimmune antibody-positive.

Fig. 5
Summarized theories of the synergistic action between natural killer (NK) cells and autoimmune antibodies causing recurrent spontaneous abortion. APA, antiphospholipid antibody; ATA, antithyroid antibody. Kim, N.Y., et al. Am J Reprod Immunol. 65(1): p. 78-87.according to the Creative Commons Attribution License [18].

Table 2
Comparison of the outcomes of the groups, which were treated with 2 weeks of low molecular weight heparin and who sustained pregnancy beyond 12 gestational weeks

AA-, autoimmune antibody-negative; nNK, normal natural killer; eNK, elevated natural killer; AA+, autoimmune antibody-positive; NS, not significant.
a)Nineteen patients had received low molecular weight heparin treatment but 2 patients had experienced spontaneous abortion and the abortus turned out to be abnormal karyotype (47, XY, +20 and 47, XY, +22) and they were excluded from the sustained pregnancy beyond 12 gestational weeks group.
References
1. Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril. 1996; 66:24–29.
2. Matthiesen L, Kalkunte S, Sharma S. Multiple pregnancy failures: an immunological paradigm. Am J Reprod Immunol. 2012; 67:334–340.
3. Tulppala M, Palosuo T, Ramsay T, Miettinen A, Salonen R, Ylikorkala O. A prospective study of 63 couples with a history of recurrent spontaneous abortion: contributing factors and outcome of subsequent pregnancies. Hum Reprod. 1993; 8:764–770.
4. Plouffe L Jr, White EW, Tho SP, Sweet CS, Layman LC, Whitman GF, et al. Etiologic factors of recurrent abortion and subsequent reproductive performance of couples: have we made any progress in the past 10 years? Am J Obstet Gynecol. 1992; 167:313–320.
5. Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006; 12:1065–1074.
6. Perricone R, Di Muzio G, Perricone C, Giacomelli R, De Nardo D, Fontana L, et al. High levels of peripheral blood NK cells in women suffering from recurrent spontaneous abortion are reverted from high-dose intravenous immunoglobulins. Am J Reprod Immunol. 2006; 55:232–239.
7. Perricone C, de Carolis C, Perricone R. Pregnancy and autoimmunity: a common problem. Best Pract Res Clin Rheumatol. 2012; 26:47–60.
8. Kwak-Kim J, Park JC, Ahn HK, Kim JW, Gilman-Sachs A. Immunological modes of pregnancy loss. Am J Reprod Immunol. 2010; 63:611–623.
9. Pratt DE, Kaberlein G, Dudkiewicz A, Karande V, Gleicher N. The association of antithyroid antibodies in euthyroid nonpregnant women with recurrent first trimester abortions in the next pregnancy. Fertil Steril. 1993; 60:1001–1005.
10. Bussen S, Steck T. Thyroid autoantibodies in euthyroid non-pregnant women with recurrent spontaneous abortions. Hum Reprod. 1995; 10:2938–2940.
11. Sladkevicius P, Valentin L, Marsal K. Blood flow velocity in the uterine and ovarian arteries during the normal menstrual cycle. Ultrasound Obstet Gynecol. 1993; 3:199–208.
12. Habara T, Nakatsuka M, Konishi H, Asagiri K, Noguchi S, Kudo T. Elevated blood flow resistance in uterine arteries of women with unexplained recurrent pregnancy loss. Hum Reprod. 2002; 17:190–194.
13. Ferreira AM, Pires CR, Moron AF, Araujo Junior E, Traina E, Mattar R. Doppler assessment of uterine blood flow in recurrent pregnancy loss. Int J Gynaecol Obstet. 2007; 98:115–119.
14. Tamura H, Miwa I, Taniguchi K, Maekawa R, Asada H, Taketani T, et al. Different changes in resistance index between uterine artery and uterine radial artery during early pregnancy. Hum Reprod. 2008; 23:285–289.
15. Choi JY, Hwang SJ, Han AR, Yoo JH, Park DW, Park CW, et al. Increased peripheral NK cell fraction and their cytolytic activity in patients with history of recurrent spontaneous abortion. Korean J Reprod Med. 2010; 37:115–124.
16. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006; 4:295–306.
17. Kwak JY, Beaman KD, Gilman-Sachs A, Ruiz JE, Schewitz D, Beer AE. Up-regulated expression of CD56+, CD56+/CD16+, and CD19+ cells in peripheral blood lymphocytes in pregnant women with recurrent pregnancy losses. Am J Reprod Immunol. 1995; 34:93–99.
18. Kim NY, Cho HJ, Kim HY, Yang KM, Ahn HK, Thornton S, et al. Thyroid autoimmunity and its association with cellular and humoral immunity in women with reproductive failures. Am J Reprod Immunol. 2011; 65:78–87.
19. Steer CV, Tan SL, Mason BA, Campbell S. Midluteal-phase vaginal color Doppler assessment of uterine artery impedance in a subfertile population. Fertil Steril. 1994; 61:53–58.
20. Goswamy RK, Williams G, Steptoe PC. Decreased uterine perfusion: a cause of infertility. Hum Reprod. 1988; 3:955–959.
21. Hui C, Lili M, Libin C, Rui Z, Fang G, Ling G, et al. Changes in coagulation and hemodynamics during pregnancy: a prospective longitudinal study of 58 cases. Arch Gynecol Obstet. 2012; 285:1231–1236.
22. El-mashad AI, Mohamed MA, Farag MA, Ahmad MK, Ismail Y. Role of uterine artery Doppler velocimetry indices and plasma adrenomedullin level in women with unexplained recurrent pregnancy loss. J Obstet Gynaecol Res. 2011; 37:51–57.
23. Frates MC, Doubilet PM, Brown DL, Benson CB, DiSalvo DN, Laing FC, et al. Role of Doppler ultrasonography in the prediction of pregnancy outcome in women with recurrent spontaneous abortion. J Ultrasound Med. 1996; 15:557–562.
24. Ng EH, Chan CC, Tang OS, Yeung WS, Ho PC. Endometrial and subendometrial vascularity is higher in pregnant patients with livebirth following ART than in those who suffer a miscarriage. Hum Reprod. 2007; 22:1134–1141.
25. Quenby S, Bates M, Doig T, Brewster J, Lewis-Jones DI, Johnson PM, et al. Pre-implantation endometrial leukocytes in women with recurrent miscarriage. Hum Reprod. 1999; 14:2386–2391.
26. Gris JC. LMWH have no place in recurrent pregnancy loss: debate-against the motion. Thromb Res. 2011; 127:Suppl 3. S110–S112.
27. Venkat-Raman N, Backos M, Teoh TG, Lo WT, Regan L. Uterine artery Doppler in predicting pregnancy outcome in women with antiphospholipid syndrome. Obstet Gynecol. 2001; 98:235–242.
28. Dolitzky M, Inbal A, Segal Y, Weiss A, Brenner B, Carp H. A randomized study of thromboprophylaxis in women with unexplained consecutive recurrent miscarriages. Fertil Steril. 2006; 86:362–366.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download