Abstract
Objective
Mesenchymal stem cells (MSC) have been considered as an ideal source of stem cells because of low immunogenicity and availability of autologous cells. Although it has been reported that amniotic membrane contains MSC, the difference between amniotic membrane-derived mesenchymal stem cells (AMSC) and MSC isolated from other tissues is still not clear. This study was designated to compare the characteristics and gene expression profi le of human AMSCs (hAMSC) and adipose tissue-derived mesenchymal stem cells (hADSC).
Methods
MSC were cultured from human amniotic membranes and adipose tissue by enzyme digestion. We compared the growth rate, surface marker expression, differentiation potential to adipogenic and osteogenic lineages, and gene expression of hAMSC with those of hADSC.
Results
hAMSC had growth rate and surface marker expression similar with hADSC. However, cyclopamine inhibited hAMSC proliferation in a dose-dependent manner without affecting hADSC proliferation. hAMSC showed lower differentiation potential to adipogenic and osteogenic lineages and lower in vivo tumor growth promoting effect in lung cancer cells xenotransplantion model of nude mouse than hADSC. Gene expression analysis using microarray revealed that many genes to be expressed differentially between hAMSC and hADSC are related to development and differentiation processes.
Figures and Tables
Fig. 1
The cell morphology hAMSC and hADSC. Photographs were taken under phase microscope (×100). hAMSC, human amniotic membrane-derived mesenchymal stem cells; hADSC, human adipose tissue-derived mesenchymal stem cells.

Fig. 2
(A) Proliferation of hAMSC and hADSC. (B) Effect of cyclopamine on the proliferation of hAMSC and hADSC. Cell number was determined 5 days after plating of cells in the presence or absence of cyclopamine. Data represent mean ± standard error of mean (SEM) of three different experiments. (C) Effect of cyclopamine on the viability of hAMSC and hADSC. Cell viability of hAMSC and hADSC in the absence or presence of cyclopamine was determined on days 4 by trypan blue exclusion assay. Data represent mean ± SEM of three different experiments. hAMSC, human amniotic membrane-derived mesenchymal stem cells; hADSC, human adipose tissue-derived mesenchymal stem cells. aP < 0.05 compared with the data in the absence of cyclopamine.

Fig. 3
Adipogenic differentiation of hAMSC and hADSC. (A) Cells were grown to confl uence and then induced to osteogenic or adipogenic differentiation in differentiation media. Adipogenic differentiation (AM) was determined with Oil Red O staining as an indicator of intracellular lipid accumulation (×200). (B) The quantitation of adipogenic differentiation was performed by measurement of optical density in isopropanol extract of oil red O staining. Data represent mean ± SEM of three different experiments. hAMSC, human amniotic membrane-derived mesenchymal stem cells; hADSC, human adipose tissue-derived mesenchymal stem cells. aP < 0.05 compared with hADSC.

Fig. 4
Osteogenic differentiation of hAMSC and hADSC. Cells were grown to confl uence and then induced to osteogenic differentiation in differentiation media. (A) Osteogenic differentiation (OM) was determined by calcification deposits on the cell monolayer, which were stained with alizarin red S Alizarin Red S. (×200). (B) The quantitation of osteogenic differentiation was performed by determination of density and area of Alizarin Red S staining with an image analysis program (Metamorph, Molecular Devices, LLC., Sunnyvale, CA, USA). Data represent mean ± standard error of mean of three different experiments. hAMSC, human amniotic membrane-derived mesenchymal stem cells; hADSC, human adipose tissue-derived mesenchymal stem cells. aP < 0.05 compared with hADSC.

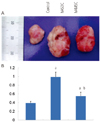
Fig. 5
Effects of hAMSC and hADSC injection on the tumor growth in vivo. (A) Effect of hASCs cotransplantation on tumor growth from xenotransplanted H460 cells. H460 (1×105) were injected subcutaneously and 1×106 hADSC or hAMSC were injected into left ventricle. Representative photograph of H460-derived tumors in nude mice on 14 days after transplantation. (B) Quantification of tumor weights. Data represent mean ± standard error of mean (n=4). hAMSC, human amniotic membrane-derived mesenchymal stem cells; hADSC, human adipose tissue-derived mesenchymal stem cells. aP < 0.05, significant difference from control data of H460 alone; bP < 0.05, significant difference from H460 + hADSC group.
References
1. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997. 276:71–74.
2. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
3. Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004. 8:301–316.
4. Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003. 102:3483–3493.
5. Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004. 40:1275–1284.
6. Tropel P, Platet N, Platel JC, Noël D, Albrieux M, Benabid AL, et al. Functional neuronal differentiation of bone marrow-derived mesenchymal stem cells. Stem Cells. 2006. 24:2868–2876.
7. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. The International Society for Cellular Therapy position statement. Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy. 2006. 8:315–317.
8. Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005. 52:2521–2529.
9. Kim Y, Kim H, Cho H, Bae Y, Suh K, Jung J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem. 2007. 20:867–876.
10. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009. 4:44–57.
11. Insausti CL, Blanquer M, Bleda P, Iniesta P, Majado MJ, Castellanos G, et al. The amniotic membrane as a source of stem cells. Histol Histopathol. 2010. 25:91–98.
12. Tamagawa T, Oi S, Ishiwata I, Ishikawa H, Nakamura Y. Differentiation of mesenchymal cells derived from human amniotic membranes into hepatocyte-like cells in vitro. Hum Cell. 2007. 20:77–84.
13. Wolbank S, Peterbauer A, Fahrner M, Hennerbichler S, van Griensven M, Stadler G, et al. Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: a comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng. 2007. 13:1173–1183.
14. Lin N, Tang Z, Deng M, Zhong Y, Lin J, Yang X, et al. Hedgehog-mediated paracrine interaction between hepatic stellate cells and marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2008. 372:260–265.
15. Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002. 16:2743–2748.
16. Valtieri M, Sorrentino A. The mesenchymal stromal cell contribution to homeostasis. J Cell Physiol. 2008. 217:296–300.
17. Yu JM, Jun ES, Bae YC, Jung JS. Mesenchymal stem cells derived from human adipose tissues favor tumor cell growth in vivo. Stem Cells Dev. 2008. 17:463–473.
18. Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009. 88:792–806.
19. Plaisant M, Fontaine C, Cousin W, Rochet N, Dani C, Peraldi P. Activation of hedgehog signaling inhibits osteoblast differentiation of human mesenchymal stem cells. Stem Cells. 2009. 27:703–713.
20. Yong RL, Shinojima N, Fueyo J, Gumin J, Vecil GG, Marini FC, et al. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009. 69:8932–8940.
21. Grisendi G, Bussolari R, Cafarelli L, Petak I, Rasini V, Veronesi E, et al. Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor-related apoptosis-inducing ligand delivery for cancer therapy. Cancer Res. 2010. 70:3718–3729.
22. Feng B, Chen L. Review of mesenchymal stem cells and tumors: executioner or coconspirator? Cancer Biother Radiopharm. 2009. 24:717–721.
23. Kuhn NZ, Tuan RS. Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J Cell Physiol. 2010. 222:268–277.
24. Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005. 19:681–693.
25. Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004. 22:233–241.
26. Dobreva G, Chahrour M, Dautzenberg M, Chirivella L, Kanzler B, Farinas I, et al. SATB2 is a multifunctional determinant of craniofacial patterning and osteoblast differentiation. Cell. 2006. 125:971–986.
27. Abdelmagid SM, Barbe MF, Arango-Hisijara I, Owen TA, Popoff SN, Safadi FF. Osteoactivin acts as downstream mediator of BMP-2 effects on osteoblast function. J Cell Physiol. 2007. 210:26–37.
28. Kveiborg M, Flyvbjerg A, Eriksen EF, Kassem M. 1,25-Dihydroxyvitamin D3 stimulates the production of insulin-like growth factor-binding proteins-2, -3 and -4 in human bone marrow stromal cells. Eur J Endocrinol. 2001. 144:549–557.
29. Mukherjee A, Rotwein P. Insulin-like growth factor-binding protein-5 inhibits osteoblast differentiation and skeletal growth by blocking insulin-like growth factor actions. Mol Endocrinol. 2008. 22:1238–1250.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download