| Korean J Leg Med. 2016 May;40(2):33-38. English. Published online May 31, 2016. https://doi.org/10.7580/kjlm.2016.40.2.33 | |
| © Copyright 2016 by the Korean Society for Legal Medicine | |
|
Gi Yeong Huh, | |
|
1Department of Forensic Medicine, Pusan National University School of Medicine, Yangsan, Korea. | |
|
2Department of Physiology, Inje University College of Medicine, Busan, Korea. | |
| Received April 30, 2016; Revised May 13, 2016; Accepted May 21, 2016. | |
|
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by- | |
|
Abstract
| |
|
Sudden unexplained nocturnal death syndrome (SUNDS) occurs predominantly in Southeast Asian people including Koreans. SUNDS is problematic for forensic pathologists because the diagnosis depends on the "exclusion of diagnosis." Moreover, the pathogenesis of SUNDS is still unclear although some cases are known to be intimately related to the Brugada syndrome. Connexin 43 (Cx43) is a principal protein of gap junction in adult cardiac myocytes, being distributed to the intercalated discs and phosphorylated in normal condition. Ischemia and hypoxia alter the expression of total Cx43 (tCx43) resulting in redistribution of non-phosphorylated Cx43 (npCx43) to the sarcoplasm or lateral cell borders of cardiac myocytes by continuing dephosphorylation. This study aimed to compare the immunoexpression pattern of Cx43 in the cardiac myocytes of SUNDS and ischemic heart disease (IHD). The study group was 26 cases of SUNDS and the control group of 24 cases of IHD with severe coronary atherosclerosis, showing no myocardial ischemic change. There was a significantly different expression of both tCx43 and npCX43 between the SUNDS and IHD group. A greater reduction in both tCx43 and npCx43 and a more delayed redistribution pattern was seen in the myocardium of SUNDS when compared with IHD. In conclusion, these results suggest that the reduced Cx43 expression in SUNDS may be inherent and indicate a risk of arrhythmia. |
|
Keywords: Brugada syndrome; Connexin 43; Immunohistochemistry; Myocardium; Myocardial ischemia |
|
|
Introduction
|
Sudden unexplained nocturnal death syndrome (SUNDS) occurs predominantly in Southeast Asian people such as Thai, Laotian, Cambodian, Filipino, Vietnamese as well as Japanese, Chinese, and Korean people. Most SUNDS cases occur at night while the healthy young males are sleeping, and no evidence explaining these deaths is seen in autopsy findings [1]. Previous genetic studies have suggested that SUNDS is most likely a disease allelic to Brugada syndrome [2]. However, the incidence of the genetic mutation related to cardiac sodium channel dysfunction including SCN5A has been found in less than 20% of SUNDS cases [3]. Therefore, SUNDS is still problematic for forensic pathologists because the diagnosis depends on the "exclusion of diagnosis."
Gap junction channels are the basic structures for intercellular communication and connection, permitting the transport of ions and molecules. In the heart, gap junction channels intervene electrical coupling between myocytes to enable the spread of electrical excitation. A gap junction channel consists of a connexon or hemichannel composed of six connexin (Cx) subunits in the plasma membrane [4]. In the adult heart, Cx43 is the major form among three isoforms of Cx; Cx40, Cx43, and Cx45 [5]. Under normal condition, Cx43 is located to the intercalated discs of cardiac myocytes as phosphorylated form. However, ischemia and hypoxia alter the expression of total Cx43 (tCx43) resulting in redistribution of non-phosphorylated Cx43 (npCx43) to the lateral borders of sarcoplamic membrane of cardiac myocytes by continuing dephosphorylation [6]. The studies suggest that the dephosphorylated and redistributed Cx43 is the early indicator of myocardial injury after hypoxia [7, 8, 9]. These studies imply that a change in Cx43 expression in the myocardium can be used to show the presence of ischemia or hypoxic insults. Cx43 expression may be a practical diagnostic marker to differentiate between ischemic and nonischemic causes of sudden cardiac death. However, there have been few studies about the specific alterations in Cx43 expression pattern in arrhythmogenic cardiac diseases such as SUNDS without ischemic or hypoxic injury.
The aim of this study was to compare the expression pattern of Cx43 in SUNDS and ischemic heart disease (IHD) and to understand the role of Cx43 in cardiac diseases without myocardial pathology that cause fatal arrhythmia.
|
Materials and Methods
|
1. Materials
The study group included SUNDS victims (SUNDS group, n=26; all males; aged 22-43 years; median age, 30.4 years), had a characteristic premortem history and negative autopsy findings [1]. The control group was individuals who died suddenly due to IHD with severe coronary atherosclerosis, showing no myocardial ischemic change (IHD group, n=24; 22 males and 2 females; aged 35-76 years; median age, 53.5 years). The control group was the same as in our previous study [9]. The survival times of the SUNDS and IHD groups were within 12 hours from the beginning of symptoms that led to their death. Witnesses, hospital, and police records were used to calculate survival times. The postmortem intervals of both study and control cases were all within 24 hours. All the cases of study and control groups were autopsied through 2006 and 2011.
2. Cx43 immunohistochemistry
The tissue slices of the left ventricle were obtained during autopsy. Six-micrometer-thick sections from formalin-fixed and paraffin-embedded blocks were cut, dried, deparaffinized, and rehydrated by standard protocol. The primary antibodies were rabbit polyclonal anti-Cx43 antibody (1:2,000, Sigma-Aldrich, St. Louis, MO, USA), which react to both phosphorylated forms of Cx3 and npCx43, and mouse monoclonal anti-npCx43 antibody (1:100, Life Technologies, Frederick, MD, USA), which detect only npCx43. The sections were incubated with a peroxide block for 30 minutes to block tissue endogenous peroxidase. The sections were incubated with 0.1% trypsin for 30 minutes at 37℃ and then with a 10% blocking serum for 30 minutes. After overnight incubation at room temperature, immunoreactions were visualized by avidin-biotin complex method (Vectastain ABC Kits, PK-4000, Vector Laboratories, Burlingame, CA, USA). Sections were incubated in diaminobezidine for color development and counterstained with hematoxylin.
Immnoexpression was counted to be positive when brown deposits were seen along the intercalated discs and lateral cell borders, regardless of intensity and sarcoplasmic staining. Expression patterns were grouped as no staining (NO), intercalated disc pattern (ID pattern, confined mostly to the intercalated discs), and redistribution pattern (R pattern, extended to the lateral borders of sarcoplasmic membrane).
3. Statistical analysis
Statistical analysis was performed using dBSTAT 5 (DBSTAT Co., Chuncheon, Korea). The difference in the tCx43 and npCx43 expression between the SUNDS and IHD group was evaluated using the Mantel-Haenszel chi-square test. The difference was considered statistically significant when a P-value was less than 0.05.
|
Results
|
1. Expression of tCx43 in the SUNDS and IHD group
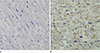
The results of tCx43 expression in the cardiac myocytes of the SUNDS and IHD group are summarized in Table 1, Fig. 1A and B. In the SUNDS group, 7/26 cases (26.9%) displayed the NO pattern; 19/26 cases (73.1%), the ID pattern. The R pattern was not observed in the SUNDS group. In the IHD group, 14/24 cases (58.3%) displayed the ID pattern; 10/24 cases (41.7%), the R pattern. The NO pattern was not observed in the IHD group. There was a significant difference in the tCx43 expression between the SUNDS and IHD group (P<0.001).
|
|
2. Expression of npCx43 in the SUNDS and IHD group
The results of npCx43 expression in the cardiac myocytes of the SUNDS and IHD group are summarized in Table 2, Fig. 2A and B. In the SUNDS group, 1/26 cases (3.8%) displayed the NO pattern; 23/26 cases (88.5%), the ID pattern; and 2/26 (7.7%), the R pattern. In the IHD group, 7/24 cases (29.2%) displayed the ID pattern; 17/24 cases (70.8%), the R pattern. There was a significant difference in the npCx43 expression between the SUNDS and IHD group (P<0.001).
|
|
|
Discussion
|
Our results showed a greater decrease in both tCx43 and npCx43 expression and a delayed pattern of redistribution in the myocardium of SUNDS when compared to IHD.
Altered Cx43 expression and distribution were reported in hypertension, cardiac hypertrophy, diabetes, hypercholesterolemia, ischemia, postmyocardial infarction remodeling, and heart failure [4, 10]. Depending on the myocardial pathology, the altered expression of Cx43 in cardiovascular diseases can occur as a decrease in expression or an altered distribution pattern. In ischemic cardiomyopathy, redistribution of Cx43 from the intercalated discs to the lateral borders of sarcoplasmic membrane is a common event [10]. During ischemia, increased intracellular Ca2+, deceased ATP, and changed phosphorylation of Cx43 cause gap junctions to close. This results in the dephosphorylayion of Cx43 and a decrease in the amount of tCx43 [11]. Using altered expression of Cx43, immunohistochemical studies have explored the use of Cx43 as a marker of early myocardial ischemic injury on forensic autopsy cases. One immunohistochemical study showed that, in the presence of myocardial ischemia, both tCx43 and npCx43 are distributed variably to the intercalated discs, cytoplasm and along the lateral border [8].
The expression of Cx43 is different in different types of cardiomyopathy. In general, Cx43 expression in the hypertrophic type appears to be unchanged or increased during the initial and compensatory phase. However, in case of prolonged hypertrophy leading to heart failure, Cx43 expression is decreased and redistributed along the lateral borders [12]. In dilated cardiomyopathy, Cx43 expression in the myocardium is markedly reduced in the 12 patients who died suddenly [13]. Additionally, in this study, there is no difference in Cx43 expression between the left and right ventricle. In arrhythmogenic right ventricular cardiomyopathy, myocardial biopsy specimens show a greater reduction in plakoglobin and Cx43 expression in the myocardium compared to dilated or hypertrophic cardiomyopathy [14]. This study suggests that a decreased expression of Cx43 and plakoglobin in the cardiac myocytes might be associated with the development of arrhythmia in arrhythmogenic right ventricular cardiomyopathy.
In pure arrhythmogenic diseases without myocardial pathology, Cx43 expression pattern is mostly reduced in the myocardium. Adult mouse model shows that induced deletion of Cx43 can cause arrhythmia by more sensitive ventricle to re-entry [15]. In this study, a reduction in Cx43 expression by about 90% results in a decrease in conduction velocity by about 50%, whereas a reduced expression by 50% induces slow conduction. Altered status of quantity, phosphorylation, and distribution of Cx43 may eventually lead to arrhythmias [16]. These studies suggest that a reduction in Cx43 expression is related to arrhythmogenesis, irrespective of the presence of myocardial pathology.
Interestingly, our study showed a greater reduction in Cx43 expression in SUNDS when compared to IHD though both conditions show sudden death. An indirect comparison was made between our results and the results of similar studies because there have been no direct immunohistochemical studies on Cx43 expression in SUNDS. In a similar study using immunofluorescence, the Cx43 signal is reduced at the right ventricular outflow tract in both autopsy and in vivo cases of Brugada syndrome, which is clinically similar to SUNDS [16]. Our result is partly consistent with the result of this study indicating a reduction in Cx43 expression in both SUNDS and Brugada syndrome. We discussed the possible reasons for a difference in Cx43 expression between SUNDS and IHD, despite of the fact that they both lead to fatal arrhythmia. Our study showed that, in the IHD group without myocardial pathology, the Cx43 redistribution to the lateral cell border occurs more frequently and earlier when compared to SUNDS. This result shows that hypoxic cardiomyocytes change the expression and phosphorylation of Cx43 in a time-dependent manner [17]. We postulate that the expression pattern of Cx43 indicates a risk of arrhythmia and is dependent on the presence of ischemic or hypoxic injury during arrhythmia. In other words, SUNDS may have an inherently low expression of Cx43 and a delayed dephosphorylation response.
The limitations of our study are as follows. First, the expression of Cx43 was not evaluated by the survival time interval from the onset of the symptom because the survival time could not be calculated precisely due to a lack of witnesses. Second, we did not perform the Cx43 expression in the right ventricle due to limited archival material. Further studies evaluating the expression of Cx43 in other arrhythmogenic diseases causing fatal arrhythmia will follow.
In conclusion, our results show a marked reduction in Cx43 expression and a delayed redistribution pattern in the myocardium of SUNDS. These findings suggest that reduced Cx43 expression in SUNDS may be inherent and indicate a risk of arrhythmia.
|
Notes
|
Conflicts of Interest:No potential conflict of interest relevant to this article was reported.
|
References
|
| 1. | Cheng J, Makielski JC, Yuan P, et al. Sudden unexplained nocturnal death syndrome in Southern China: an epidemiological survey and SCN5A gene screening. Am J Forensic Med Pathol 2011;32:359–363. |
| 2. | Vatta M, Dumaine R, Varghese G, et al. Genetic and biophysical basis of sudden unexplained nocturnal death syndrome (SUNDS), a disease allelic to Brugada syndrome. Hum Mol Genet 2002;11:337–345. |
| 3. | Liu C, Tester DJ, Hou Y, et al. Is sudden unexplained nocturnal death syndrome in Southern China a cardiac sodium channel dysfunction disorder? Forensic Sci Int 2014;236:38–45. |
| 4. | Schulz R, Gorge PM, Gorbe A, et al. Connexin 43 is an emerging therapeut ic target in i schemia/ reper fus ion injury, cardioprotection and neuroprotection. Pharmacol Ther 2015;153:90–106. |
| 5. | Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol 2004;36:1171–1186. |
| 6. | Beardslee MA, Lerner DL, Tadros PN, et al. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res 2000;87:656–662. |
| 7. | Matsushita S, Kurihara H, Watanabe M, et al. Alterations of phosphorylation state of connexin 43 during hypoxia and reoxygenation are associated with cardiac function. J Histochem Cytochem 2006;54:343–353. |
| 8. | Kawamoto O, Michiue T, Ishikawa T, et al. Immunohistochemistry of connexin43 and zonula occludens-1 in the myocardium as markers of early ischemia in autopsy material. Histol Histopathol 2014;29:767–775. |
| 9. | Ahn JW, Huh GY. The efficacy of connexin 43 expression in the myocardium as an early ischemic marker in forensic autopsy. Korean J Leg Med 2015;39:6–11. |
| 10. | Michela P, Velia V, Aldo P, et al. Role of connexin 43 in cardiovascular diseases. Eur J Pharmacol 2015;768:71–76. |
| 11. | Johansen D, Cruciani V, Sundset R, et al. Ischemia induces closure of gap junctional channels and opening of hemichannels in heart-derived cells and tissue. Cell Physiol Biochem 2011;28:103–114. |
| 12. | Teunissen BE, Jongsma HJ, Bierhuizen MF. Regulation of myocardial connexins during hypertrophic remodelling. Eur Heart J 2004;25:1979–1989. |
| 13. | Chen X, Zhang Y. Myocardial Cx43 expression in the cases of sudden death due to dilated cardiomyopathy. Forensic Sci Int 2006;162:170–173. |
| 14. | Yoshida T, Kawano H, Kusumoto S, et al. Relationships between clinical characteristics and decreased plakoglobin and connexin 43 expressions in myocardial biopsies from patients with arrhythmogenic right ventricular cardiomyopathy. Int Heart J 2015;56:626–631. |
| 15. | van Rijen HV, Eckardt D, Degen J, et al. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation 2004;109:1048–1055. |
| 16. | Nademanee K, Raju H, de Noronha SV, et al. Fibrosis, connexin-43, and conduction abnormalities in the brugada syndrome. J Am Coll Cardiol 2015;66:1976–1986. |
| 17. | Hatanaka K, Kawata H, Toyofuku T, et al. Down-regulation of connexin43 in early myocardial ischemia and protective effect by ischemic preconditioning in rat hearts in vivo. Jpn Heart J 2004;45:1007–1019. |




 ePub
ePub Citation
Citation Print
Print