Abstract
Connexin-43, a major gap junction protein, and cytokeratin-19, one of the intermediate filament keratins, are known to be markers of well-differentiated epithelium. In this study, we investigated the expression of these markers in the head region, lungs, and abdominal organs of 10 human mid-term fetuses. The expression of connexin-43 was found to be restricted to the dura mater, kidney, and adrenal cortex. In the kidney, we found a clear site-dependent difference in the expression pattern of these markers: connexin-43 expression was observed in the tubules of the renal cortex whereas cytokeratin-19 was strongly expressed in the collecting ducts and renal pelvis. This difference remained unchanged throughout the fetal stages examined. Immunoreactivity was not observed for either of the markers in the intrarenal vessels, including the glomeruli, and mesangial cells. Connexin-43 expression seemed to be restricted to the metanephric vesicle-derived structures that differentiate in the urogenital ridge of the splanchnic mesoderm. The adrenal cortex also originates from the same para-aortic mesoderm. In contrast, in the urogenital organs, cytokeratin-19 seemed to be expressed in ducts derived from the urogenital sinus.
Connexin-43 (CX43) is a well-known major subunit of gap junction channels and is expressed in the epidermis, brain, pituitary, heart, lungs, muscles, kidneys, gut, adrenal cortex, and dura mater in mouse fetuses [1, 2], especially the latter two sites. Since the initial descriptions of CX43, the distribution of and changes in CX43 expression during fetal development, regeneration, and pathological processes have been studied in the brain [3, 4], spinal cord [5], retina [6], lens [7, 8], cornea [9], heart [10], lung [11], kidney [12-14], tendons [15, 16], cartilage [17], and hair follicles [18]. In the kidney, CX43 is expressed in the vascular wall and mesangium, as well as the tubules [12]. It has been shown that CX43 is expressed in the tubules of the adult human kidney and that its expression is lost as a result of neoplasia; however, only a limited number of studies have been performed in this regard [19].
Cytokeratins or keratins constitute a group of intermediate filament proteins present in cells. Cytokeratin-19 (CK19) is commonly used as a marker of most neuroendocrine and gastrointestinal tumors [20]. CK19 is also known to be a stem cell marker in the liver [21, 22], pancreas [23], and epidermis [24]. In contrast, cytokeratin-14 (CK14), although not as well known as CK19, is used as a marker of the oral epithelium [25-27] and is considered to be a marker of maturation of the epithelial lining [26-28]. The CK14 gene is often used as a promoter in experimental systems employing dermalepidermal cells [29, 30]. Recently, our group demonstrated that liver stem cell candidates in human adults and fetuses were both positive for CK14 and CK19 [31].
Although numerous studies have been performed on CX43, CK14, and CK19, none appear to have addressed the spatial relationship between these groups of important proteins in the epithelial and endothelial linings. Therefore, the aim of this study was to investigate the expression of CX43, CK14, and CK19 in abdominal viscera, including the lungs, in human mid-term fetuses. Head specimens were also included as positive controls for CX43 immunoreactivity because strong CX43 expression has been reported in the dura (see above).
This study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). We performed histological analysis of paraffin-embedded sections from 10 mid-term fetuses at an estimated gestational age of 10-16 weeks (crown-rump length [CRL], 50-120 mm). The 10 fetuses included 2 fetuses at 10 weeks (CRL, 50-58 mm), 3 at 12 weeks (CRL, 71-80 mm), and 5 at 15-16 weeks (CRL, 102-120 mm). These specimens had been donated to the Department of Anatomy, Chonbuk National University, Korea, with the agreement of the families concerned, and their use for research had been approved by the university ethics committee. In accordance with university or hospital regulations, authors other than those affiliated with Chonbuk University were not required to submit details of this research project to the corresponding committee in Japan. All the fetuses had been obtained by induced abortion. Subsequently, each of the mothers concerned had been personally informed by an obstetrician about the possibility of donating the fetus for research; no attempt had been made to encourage donation. Because the specimens were numbered randomly, it was not possible to trace any of the families concerned.
The donated fetuses were fixed with 10% (w/w) neutral formalin solution for more than 3 months. After division into the head and neck, thorax, abdomen, pelvis, and the 4 extremities, all the parts were decalcified by incubating them at 4℃ in 0.5 mol/l ethylenediaminetetraacetic acid solution (pH 7.5, decalcifying solution B; Wako, Tokyo, Japan) for 1-3 days depending on the size of the material. Routine procedures for histological analysis (yielding sections that were 5 µm thick) of paraffin-embedded sections were conducted. All specimens of the head and the abdomen, including the lower part of the lung, were processed into sagittal sections cut at 10 to 100 µm intervals depending on the specimen size. Most sections were stained with hematoxylin and eosin, while some were used for immunohistochemistry (see below).
The primary antibodies used for immunohistochemistry were 1) rabbit polyclonal anti-human CX43 (1:100, #3512S, Cell Signaling Technology, Beverly, MA, USA), 2) mouse monoclonal anti-human CK14 (1:50, #LL002, Novo, Newcastle upon Tyne, UK), and 3) mouse monoclonal antihuman CK19 (1:100, #sc-6278, Santa Cruz Biotechnology, Santa Cruz, CA, USA). The secondary antibody (subjected to incubation for 30 minutes; Histofine Simple Stain Max-PO, Nichirei, Tokyo, Japan) was labeled with horseradish peroxidase (HRP), and antigen-antibody reactions were detected by the HRP-catalyzed reaction with diaminobenzidine (incubation for 3-5 minutes; Histofine Simple Stain DAB, Nichirei). All samples were counterstained with hematoxylin. Negative controls consisted of samples without the primary antibody.
CK19 was expressed in the epithelial linings of 1) the bronchi of the lung, 2) the esophagus, 3) the gallbladder and thick bile ducts, 4) the ureter, renal pelvis, and collecting ducts of the kidney, and 5) the colon and appendix (Fig. 1). The highest level of immunoreactivity was seen in the kidney and ureter, as well as in the squamous epithelium of the esophagus. Negative results were obtained for CK14 immunoreactivity in the abdomen, except for the skin. However, in the head, both CK14 and CK19 were strongly expressed in the epithelial linings of the oral cavity, upper pharynx, external ear, and conjunctiva (data not shown). CK19 was also expressed in the lining of the developing cochlear duct of the ear.
Weak positive results were obtained for CX43 immunoreactivity in the colon and kidney and strongly positive immunoreactivity was noted in the adrenal cortex (Fig. 1). In the head, CX43 was expressed in the dura mater, especially along the skull base (data not shown). The expression of CK19 in the kidney was restricted to the collecting ducts, whereas CX43 immunoreactivity was absent in the ducts. Conversely, CX43 was apparently expressed in the distal and proximal tubule systems (Figs. 2, 3). The immunoreactivity of CX43 was restricted to specific parts of the tubules at 10-11 weeks (Fig. 3A, C), but it extended to almost all tubules in the medulla by 15-16 weeks (Fig. 2A, C). All the vessels, including the renal arterioles and glomeruli, and mesangial cells yielded negative results for CX43. These patterns of immunoreactivity did not differ among the developmental stages examined: CX43 expression did not extend to the collecting duct in the larger specimens (Fig. 2).
We considered CX43 and CK19 as representative epithelial markers although their cytological roles seemed to be quite different (see Introduction). CX43 was not expressed in the renal arterioles even though it is known to be one of the vascular connexins that is present in the kidney, possibly because of the relatively poor state of preservation of the human fetuses before fixation in this study [12, 32, 33]. Guo et al. [34] described details of the distribution of CX43 in the adult rat kidney: the inner medullary collecting ducts showed the highest level of expression, followed by the cortical collecting ducts; this was in contrast to the significantly lower expression that was observed in the proximal convoluted tubules, proximal straight tubules, medullary thick ascending limb, and distal convoluted tubules. Stoessel et al. [35] also detected CX43 in parts of the tubular epithelial gap junctions and/or hemichannels in rats. In the human fetuses used in the current study, identification of each part of the tubule system was difficult, but the CX43 immunoreactivity appeared to be restricted to the tubules and did not extend to the collecting ducts. Therefore, it was suggested that CX43 expression was restricted to the metanephric vesicle-derived structures that differentiate in the urogenital ridge of the splanchnic mesoderm [36, 37]. The adrenal cortex also originates from the same para-aortic part of the splanchnic mesoderm [36, 37]; in fact, in the current study, the adrenal cortex was the only organ that was strongly positive for CX43.
Despite the fact that CK14 has been widely used as an epithelial marker in studies on the maxillofacial region (see Introduction), it did not appear to be expressed in other parts of the gastrointestinal tract in the human fetuses used in the current study. Likewise, CK19 immunoreactivity was strong in the squamous epithelium such as that of the esophagus, but it was weak in columnar epithelium such as that of the colon. Despite being similar to CX43, in this study, CK19 was not expressed in the renal glomeruli, although Oosterwijk et al. [38] had previously reported CK19 reactivity in parietal glomerular cells of the human fetal kidneys at 11-20 weeks of gestation. This was possibly due to the relatively poor state of the preserved specimens. In the current study, strong positive results were obtained for CK19 in the ureter, renal pelvis, and collecting ducts. Thus, its expression was restricted to the urinary tract derived from the ureteric bud of the urogenital sinus [36, 37]. This finding was in clear contrast to the expression of CX43 in the urinary organs. In fact, the Műllerian duct does not express CK14 [39]. Therefore, unlike CX43-positive tissues (see the paragraph above), the urogenital ridge derivatives may be negative for CK. Oosterwijk et al. [38] reported CK19 expression in the human fetal distal tubule and Henle's loop at 11-20 weeks of gestation. These portions are also likely to originate from the ureteric bud. In that paper, the authors included images of a CK19-positive S-shaped body primitive morphological feature of the tubule system. However, we believe that the structure had already differentiated into the mature tubules and disappeared at 11 weeks.
Consequently, the special relationship that we observed between the expression of CX43 and that of CK19 appeared to reflect the ontogenic origins of the tissues rather than the functional differentiation of the tubule and duct. The segment-specific expression patterns of the tight junction proteins, claudins, have been well described in the kidney tubule and duct [40]. Therefore, the CK19-positive collecting duct is likely to contain a connexin other than CX43, whereas the CX43-positive tubule is likely to be reinforced by a cytokeratin other than CK19 and CK14. CK19 is known to be expressed strongly in some renal neoplasms such as papillary renal cell carcinoma and mucinous tubular and spindle cell carcinoma [41, 42]. These carcinomas may originate from the collecting ducts or renal pelvis.
Figures and Tables
 | Fig. 1Immunohistochemical analysis of cytokeratin and connexin in the human fetal lung and abdominal organs: a fetus at 12 weeks. (A, D, G, J) Expression of cytokeratin-19 (CK19); (B, E, H, K) expression of cytokeratin-14 (CK14); (C, F, I, L) expression of connexin-43 (CX43). CK19 immunopositivity is seen in the lung (A), kidney (D), colon (G), and gallbladder (J), whereas these tissues lack CK14 immunoreactivity. The kidney and adrenal cortex (F) show positive results for CX43. Scale bar in (C)=0.5 mm (A-L). |
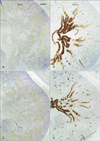 | Fig. 2Immunohistochemical results for cytokeratin and connexin in the human fetal kidney: 2 fetuses at 16 weeks. Panels (A) and (B) and panels (C) and (D) display adjacent sections of the same specimen. Connexin-43 (CX43) immunopositivity is seen in the renal tubules of the cortex (A, C), whereas strong immunoreactivity is seen for cytokeratin-19 (CK19) in the collecting ducts and renal pelvis (B, D). Scale bar in (A)=0.5 mm (A-D). |
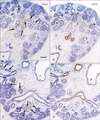 | Fig. 3Immunohistochemical analysis for cytokeratin and connexin in the human fetal kidney: a fetus at 11 weeks. Panels (A) and (B) and panels (C) and (D) show adjacent sections. Connexin-43 (CX43) immunopositivity is seen in some of the renal tubules of the cortex (arrows in A and C), whereas cytokeratin-19 (CK19) shows strong immunoreactivity in the collecting ducts and renal pelvis (B, D). Scale bar in (A)=0.2 mm (A-D). |
Acknowledgements
This study was supported by a grant (0620220-1) from the National R & D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea.
References
1. Dahl E, Winterhager E, Traub O, Willecke K. Expression of gap junction genes, connexin40 and connexin43, during fetal mouse development. Anat Embryol (Berl). 1995. 191:267–278.
2. Yancey SB, Biswal S, Revel JP. Spatial and temporal patterns of distribution of the gap junction protein connexin43 during mouse gastrulation and organogenesis. Development. 1992. 114:203–212.
3. Kunze A, Congreso MR, Hartmann C, Wallraff-Beck A, Hüttmann K, Bedner P, Requardt R, Seifert G, Redecker C, Willecke K, Hofmann A, Pfeifer A, Theis M, Steinhäuser C. Connexin expression by radial glia-like cells is required for neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2009. 106:11336–11341.
4. Leung DS, Unsicker K, Reuss B. Expression and developmental regulation of gap junction connexins cx26, cx32, cx43 and cx45 in the rat midbrain-floor. Int J Dev Neurosci. 2002. 20:63–75.
5. Lee IH, Lindqvist E, Kiehn O, Widenfalk J, Olson L. Glial and neuronal connexin expression patterns in the rat spinal cord during development and following injury. J Comp Neurol. 2005. 489:1–10.
6. Kerr NM, Johnson CS, de Souza CF, Chee KS, Good WR, Green CR, Danesh-Meyer HV. Immunolocalization of gap junction protein connexin43 (GJA1) in the human retina and optic nerve. Invest Ophthalmol Vis Sci. 2010. 51:4028–4034.
7. Long AC, Bomser JA, Grzybowski DM, Chandler HL. Alltrans retinoic acid regulates cx43 expression, gap junction communication and differentiation in primary lens epithelial cells. Curr Eye Res. 2010. 35:670–679.
8. Long AC, Colitz CM, Bomser JA. Regulation of gap junction intercellular communication in primary canine lens epithelial cells: role of protein kinase C. Curr Eye Res. 2007. 32:223–231.
9. Wolosin JM, Schütte M, Zieske JD, Budak MT. Changes in connexin43 in early ocular surface development. Curr Eye Res. 2002. 24:430–438.
10. Coppen SR, Kaba RA, Halliday D, Dupont E, Skepper JN, Elneil S, Severs NJ. Comparison of connexin expression patterns in the developing mouse heart and human foetal heart. Mol Cell Biochem. 2003. 242:121–127.
11. Nagata K, Masumoto K, Esumi G, Teshiba R, Yoshizaki K, Fukumoto S, Nonaka K, Taguchi T. Connexin43 plays an important role in lung development. J Pediatr Surg. 2009. 44:2296–2301.
12. McCulloch F, Chambrey R, Eladari D, Peti-Peterdi J. Localization of connexin 30 in the luminal membrane of cells in the distal nephron. Am J Physiol Renal Physiol. 2005. 289:F1304–F1312.
13. Toubas J, Beck S, Pageaud AL, Huby AC, Mael-Ainin M, Dussaule JC, Chatziantoniou C, Chadjichristos CE. Alteration of connexin expression is an early signal for chronic kidney disease. Am J Physiol Renal Physiol. 2011. 301:F24–F32.
14. Udaka N, Ito T, Sato Y, Satoh S, Kanisawa M. Expression of connexin 32 gap junction protein in the kidneys during fetal development of the hamster (Mesocricetus auratus). Anat Embryol (Berl). 1995. 192:399–406.
15. Stanley RL, Fleck RA, Becker DL, Goodship AE, Ralphs JR, Patterson-Kane JC. Gap junction protein expression and cellularity: comparison of immature and adult equine digital tendons. J Anat. 2007. 211:325–334.
16. Young NJ, Becker DL, Fleck RA, Goodship AE, Patterson-Kane JC. Maturational alterations in gap junction expression and associated collagen synthesis in response to tendon function. Matrix Biol. 2009. 28:311–323.
17. Loty S, Foll C, Forest N, Sautier JM. Association of enhanced expression of gap junctions with in vitro chondrogenic differentiation of rat nasal septal cartilage-released cells following their dedifferentiation and redifferentiation. Arch Oral Biol. 2000. 45:843–856.
18. Arita K, Akiyama M, Tsuji Y, McMillan JR, Eady RA, Shimizu H. Gap junction development in the human fetal hair follicle and bulge region. Br J Dermatol. 2004. 150:429–434.
19. Wilgenbus KK, Kirkpatrick CJ, Knuechel R, Willecke K, Traub O. Expression of Cx26, Cx32 and Cx43 gap junction proteins in normal and neoplastic human tissues. Int J Cancer. 1992. 51:522–529.
20. Jain R, Fischer S, Serra S, Chetty R. The use of cytokeratin 19 (CK19) immunohistochemistry in lesions of the pancreas, gastrointestinal tract, and liver. Appl Immunohistochem Mol Morphol. 2010. 18:9–15.
21. Allain JE, Dagher I, Mahieu-Caputo D, Loux N, Andreoletti M, Westerman K, Briand P, Franco D, Leboulch P, Weber A. Immortalization of a primate bipotent epithelial liver stem cell. Proc Natl Acad Sci U S A. 2002. 99:3639–3644.
22. Golbar HM, Izawa T, Murai F, Kuwamura M, Yamate J. Immunohistochemical analyses of the kinetics and distribution of macrophages, hepatic stellate cells and bile duct epithelia in the developing rat liver. Exp Toxicol Pathol. 2012. 64:1–8.
23. Yao ZX, Qin ML, Liu JJ, Chen XS, Zhou DS. In vitro cultivation of human fetal pancreatic ductal stem cells and their differentiation into insulin-producing cells. World J Gastroenterol. 2004. 10:1452–1456.
24. Liu HW, Cheng B, Li JF, Wu HJ, Li KY, Sun TZ, Fu XB. Characterization of angiotensin-converting enzyme expression during epidermis morphogenesis in humans: a potential marker for epidermal stem cells. Br J Dermatol. 2009. 160:250–258.
25. Iwasaki S, Aoyagi H, Yoshizawa H. Localization of keratins 13 and 14 in the lingual mucosa of rats during the morphogenesis of circumvallate papillae. Acta Histochem. 2011. 113:395–401.
26. Lourenco SV, Coutinho-Camillo CM, Buim ME, Uyekita SH, Soares FA. Human salivary gland branching morphogenesis: morphological localization of claudins and its parallel relation with developmental stages revealed by expression of cytoskeleton and secretion markers. Histochem Cell Biol. 2007. 128:361–369.
27. Lourenço SV, Lima DM, Uyekita SH, Schultz R, de Brito T. Expression of beta-1 integrin in human developing salivary glands and its parallel relation with maturation markers: in situ hybridisation and immunofluorescence study. Arch Oral Biol. 2007. 52:1064–1071.
28. Martins MD, Cavalcanti de Araujo V, Raitz R, Soares de Araújo N. Expression of cytoskeletal proteins in developing human minor salivary glands. Eur J Oral Sci. 2002. 110:316–321.
29. Cobourne MT, Xavier GM, Depew M, Hagan L, Sealby J, Webster Z, Sharpe PT. Sonic hedgehog signalling inhibits palatogenesis and arrests tooth development in a mouse model of the nevoid basal cell carcinoma syndrome. Dev Biol. 2009. 331:38–49.
30. Mayer JA, Foley J, De La Cruz D, Chuong CM, Widelitz R. Conversion of the nipple to hair-bearing epithelia by lowering bone morphogenetic protein pathway activity at the dermal-epidermal interface. Am J Pathol. 2008. 173:1339–1348.
31. Moon WS, Cho BH, Hayashi S, Kim JH, Murakami G, Fukuzawa Y, Nakano T. Cytokeratin-positive hepatocytes in the hilar region: an immunohistochemical study using livers from fetuses and elderly individuals. Ann Anat. 2011. 193:224–230.
32. Kurtz L, Madsen K, Kurt B, Jensen BL, Walter S, Banas B, Wagner C, Kurtz A. High-level connexin expression in the human juxtaglomerular apparatus. Nephron Physiol. 2010. 116:1–8.
33. Takenaka T, Inoue T, Kanno Y, Okada H, Meaney KR, Hill CE, Suzuki H. Expression and role of connexins in the rat renal vasculature. Kidney Int. 2008. 73:415–422.
34. Guo R, Liu L, Barajas L. RT-PCR study of the distribution of connexin 43 mRNA in the glomerulus and renal tubular segments. Am J Physiol. 1998. 275(2 Pt 2):R439–R447.
35. Stoessel A, Himmerkus N, Bleich M, Bachmann S, Theilig F. Connexin 37 is localized in renal epithelia and responds to changes in dietary salt intake. Am J Physiol Renal Physiol. 2010. 298:F216–F223.
36. Hamilton WJ, Mossman HW. Hamilton, Boyd and Mossman's human embryology: prenatal development of form and function. 1978. Cambridge: Heffer;384–391.
37. O'Rahilly RR, Müller F. Human embryology and teratology. 1996. 2nd ed. New York: Wiley-Liss;274–277.
38. Oosterwijk E, Van Muijen GN, Oosterwijk-Wakka JC, Warnaar SO. Expression of intermediate-sized filaments in developing and adult human kidney and in renal cell carcinoma. J Histochem Cytochem. 1990. 38:385–392.
39. Kenngott RA, Sinowatz F. Expression and distribution of intermediate-filament proteins and laminin during the development of the bovine Müllerian duct. Anat Histol Embryol. 2008. 37:223–230.
40. Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002. 13:875–886.
41. Kim MK, Kim S. Immunohistochemical profile of common epithelial neoplasms arising in the kidney. Appl Immunohistochem Mol Morphol. 2002. 10:332–338.
42. Truong LD, Shen SS. Immunohistochemical diagnosis of renal neoplasms. Arch Pathol Lab Med. 2011. 135:92–109.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download