Abstract
Purpose
Materials and Methods
Results
Figures and Tables
Fig. 1
Ultrasound (US) of group A and group B.

Fig. 2
Ultrasound (US) of group Ho and group He.
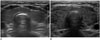
Fig. 3
Ultrasound (US) of group 1, 2, and 3.

Table 1
Epidemiologic and Sonographic Characteristics of 543 Patients with Decreased Echogenicity

Group A: patients with lower echogenicity than that of submandibular gland, but higher than that of strap muscle, Group B: patients with isoechoic or lower echogenicity than that of strap muscle, Group Ho: patients with homogeneous decreased echogenicity of thyroid, Group He: patients with multiple scattered small hypoechoic nodules in whole thyroid parenchyma, Group 1: decreased size, Group 2: normal size, Group 3: increased size
Table 2
Thyroid Function and Autoimmune Activity of 543 Patients with Decreased Echogenicity

Reference value: T3 (0.89–1.68 ng/mL), free T4 (0.88–1.44 ng/dL), TSH (0.35–5.50 uIU/mL), TgAb (~40 IU/mL), TPOAb (~35 IU/mL), TSH-rAb (~1.75 IU/L).
fT4 = free-thyroxine, TgAb = anti-thyroglobulin antibody, TPOAb = thyroid peroxidase antibody, TSH = thyrotropin, TSH-rAb = anti-TSH receptor antibody, T3 = triiodiothyronyne
Table 3
Comparison of Thyroid Function Test or Thyroid Autoantibodies between Mildly Decreased Echogenicity (Group A) and Markedly Decreased Echogenicity (Group B)

Group A: patients with lower echogenicity than that of submandibular gland, but higher than that of strap muscle, Group B: patients with isoechoic or lower echogenicity than that of strap muscle.
*Pearson chi-square test was used.
fT4 = free-thyroxine, TgAb = anti-thyroglobulin antibody, TPOAb = thyroid peroxidase antibody, TSH = thyrotropin, TSH-rAb = anti-TSH receptor antibody, T3 = triiodiothyronyne
Table 4
Comparison of Thyroid Function Test or Thyroid Autoantibodies between Homogeneous (Group Ho) and Heterogeneous Group (Group He)

Group Ho: patients with homogeneous decreased echogenicity of thyroid, Group He: patients with multiple scattered small hypoechoic nodules in whole thyroid parenchyma.
*Pearson chi-square test was used.
fT4 = free-thyroxine, TgAb = anti-thyroglobulin antibody, TPOAb = thyroid peroxidase antibody, TSH = thyrotropin, TSH-rAb = anti-TSH receptor antibody, T3 = triiodiothyronyne
Table 5
Comparison of Thyroid Function Test or Thyroid Autoantibodies among Decreased (Group 1), Normal (Group 2), and Increased Size of Thyroid (Group 3)





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download