Abstract
Because papillary thyroid carcinoma (PTC) is indolent and has an excellent prognosis, active surveillance, without immediate surgery, can be considered for small PTC. However, rarely, PTC can transform to anaplastic thyroid carcinoma (ATC), over a period of 5–20 years. We report 73-year-old man with rapid anaplastic transformation of a PTC. He was diagnosed with colorectal cancer, and a 1-cm-sized thyroid nodule was found incidentally and confirmed as PTC on fine-needle aspiration. He underwent transanal excision and chemotherapy for colorectal cancer. However, he was not concerned about the PTC, and no follow-up examination was performed. After 37 months, he suddenly noticed an enlarging neck mass, which was diagnosed as an ATC. Despite total thyroidectomy, locally advanced recurrence with lung metastasis developed, and he eventually died. Although PTC is indolent and progresses slowly in elderly people, it can transform to ATC. Therefore, during active surveillance in the elderly, follow-up examinations should be performed regularly.
References
1. Nilubol N, Kebebew E. Should small papillary thyroid cancer be observed? A population-based study. Cancer. 2015; 121(7):1017–24.

2. Sugitani I, Toda K, Yamada K, Yamamoto N, Ikenaga M, Fujimoto Y. Three distinctly different kinds of papillary thyroid microcarcinoma should be recognized: our treatment strategies and outcomes. World J Surg. 2010; 34(6):1222–31.

3. Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010; 34(1):28–35.

4. Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014; 24(1):27–34.

5. Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003; 237(3):399–407.
6. Chow SM, Law SC, Chan JK, Au SK, Yau S, Lau WH. Papillary microcarcinoma of the thyroid-prognostic significance of lymph node metastasis and multifocality. Cancer. 2003; 98(1):31–40.

7. Hay ID, Hutchinson ME, Gonzalez-Losada T, McIver B, Reinalda ME, Grant CS, et al. Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period. Surgery. 2008; 144(6):980–7. ; discussion 7–8.

8. Cho SY, Lee TH, Ku YH, Kim HI, Lee GH, Kim MJ. Central lymph node metastasis in papillary thyroid microcarcinoma can be stratified according to the number, the size of metastatic foci, and the presence of desmoplasia. Surgery. 2015; 157(1):111–8.

9. Oh CM, Jung KW, Won YJ, Shin A, Kong HJ, Lee JS. Age-period-cohort analysis of thyroid cancer incidence in Korea. Cancer Res Treat. 2015; 47(3):362–9.

10. Wiseman SM, Loree TR, Rigual NR, Hicks WL Jr, Douglas WG, Anderson GR, et al. Anaplastic transformation of thyroid cancer: review of clinical, pathologic, and molecular evidence provides new insights into disease biology and future therapy. Head Neck. 2003; 25(8):662–70.

11. McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, et al. Anaplastic thyroid carcinoma: a 50-year experience at a single institution. Surgery. 2001; 130(6):1028–34.

12. Venkatesh YS, Ordonez NG, Schultz PN, Hickey RC, Goepfert H, Samaan NA. Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer. 1990; 66(2):321–30.

13. Lee DY, Won JK, Lee SH, Park do J, Jung KC, Sung MW, et al. Changes of clinicopathologic characteristics and survival outcomes of anaplastic and poorly differentiated thyroid carcinoma. Thyroid. 2016; 26(3):404–13.

14. Yoshida A, Sugino K, Sugitani I, Miyauchi A. Anaplastic thyroid carcinomas incidentally found on postoperative pathological examination. World J Surg. 2014; 38(9):2311–6.

15. Han JM, Bae Kim W, Kim TY, Ryu JS, Gong G, Hong SJ, et al. Time trend in tumour size and characteristics of anaplastic thyroid carcinoma. Clin Endocrinol (Oxf). 2012; 77(3):459–64.

16. Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer. 2009; 16(1):17–44.

17. Ahn HS, Kim HJ, Welch HG. Korea's thyroid-cancer "epidemic"–screening and overdiagnosis. N Engl J Med. 2014; 371(19):1765–7.
18. Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006; 295(18):2164–7.

19. Kaushal S, Sharma MC, Mathur SR, Rastogi S, Bal CS, Chumber S. Anaplastic transformation of metastatic papillary thyroid carcinoma at shoulder mimicking soft tissue sarcoma. Indian J Pathol Microbiol. 2011; 54(4):796–9.
20. Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid. 2011; 21(2):125–34.

21. Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol. 2006; 13(4):453–64.

22. Kim TY, Kim KW, Jung TS, Kim JM, Kim SW, Chung KW, et al. Prognostic factors for Korean patients with anaplastic thyroid carcinoma. Head Neck. 2007; 29(8):765–72.

Fig. 1.
Images and pathologic findings of PTC at the time of initial diagnosis. (A) PET-CT image showed a hypermetabolic mass (SUV 22.9) in the right thyroid gland. (B) Neck US showed a 1.0-cm round, rim calcified nodule in the right thyroid gland. (C) Specimen obtained by US-guided core needle biopsy and tumor cells showed typical morphology of PTC such as intranuclear inclusion, ground glass ap-pearance of nuclei and papillary growth pattern (H&E staining, original magnification ×200).
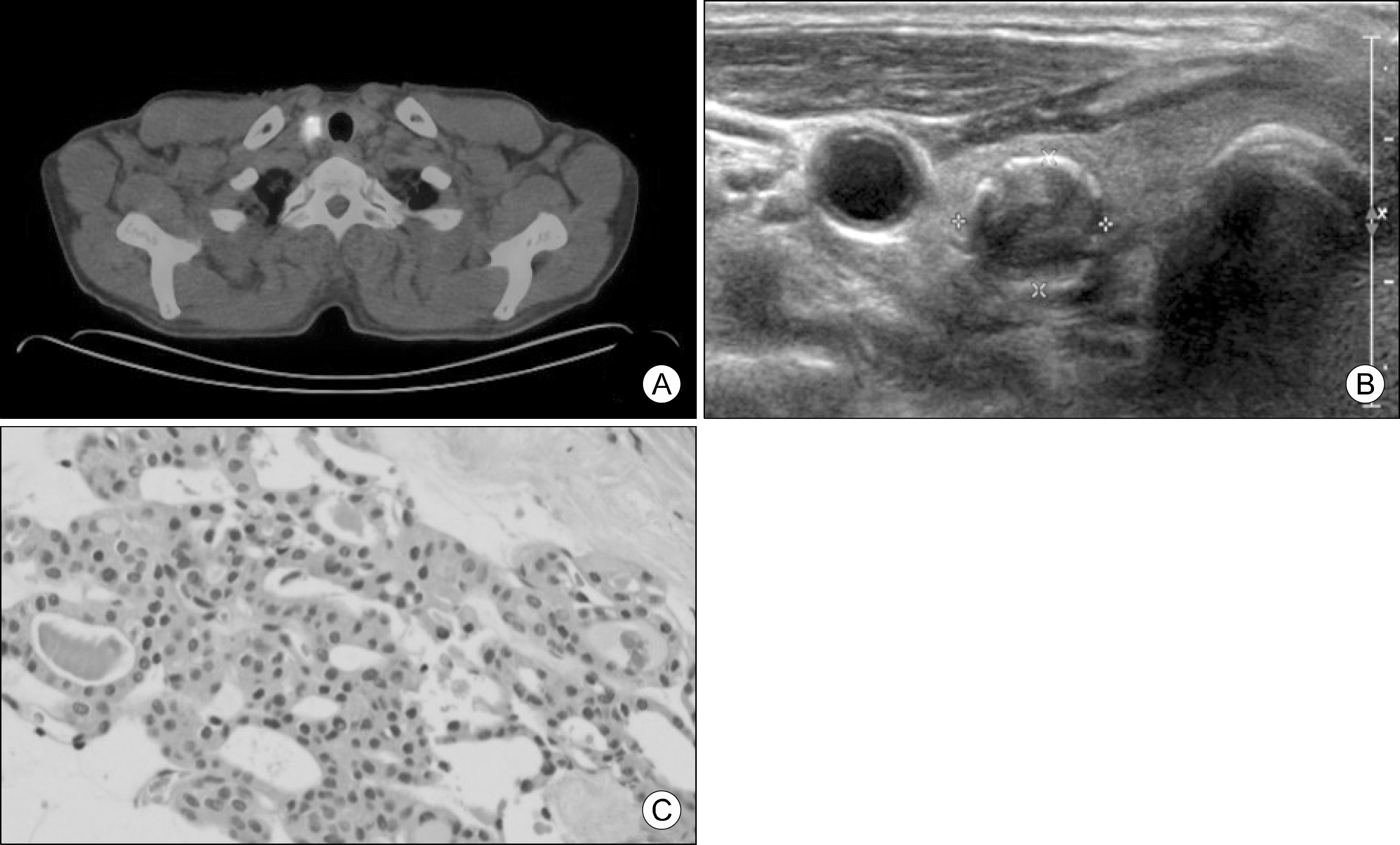
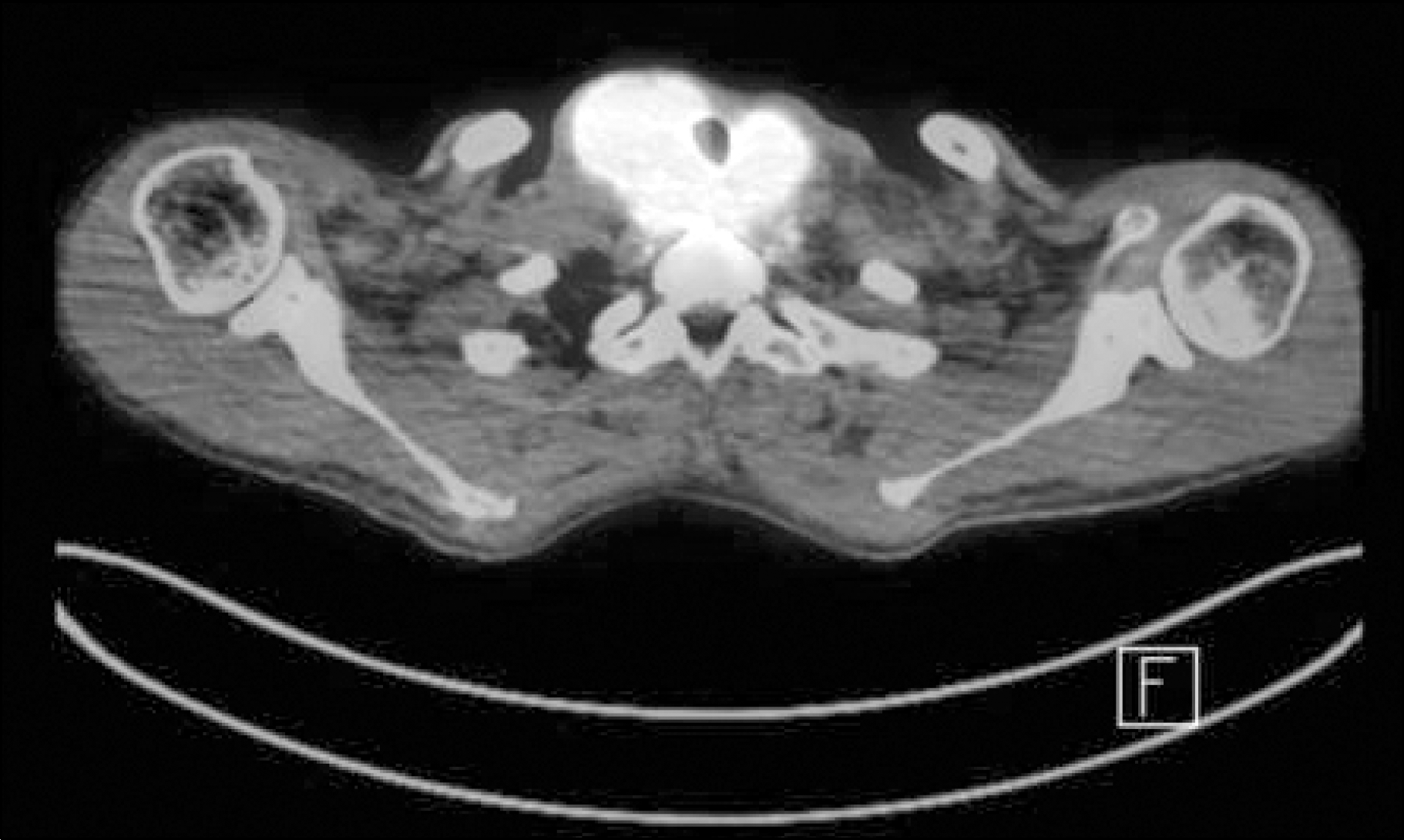
Fig. 2.
Images and pathologic findings after anaplastic trans-formation. (A) Neck US showed a 4.8-cm solid and calcified nodule in the right thyroid gland. (B) PET-CT image showed a hypermetabolic mass (SUV 29.0) in the right thyroid gland. (C) Specimen obtained by surgery and it revealed typical morphology of ATC that is patternless pleomorphic spindle cells with high mitotic count (H&E staining, original magnification ×200).





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download