Introduction
Zinc (Zn) is an essential trace element which is required for metabolism, signal transduction, cell growth and differentiation. It is a critical component of many proteins and enzymes, including Zn-dependent transcription factors, and it is involved in DNA synthesis, RNA transcription, and cell division and activation.12 Zinc deficiency affects many organ systems, including the integumentary, gastrointestinal, central nervous, immune, skeletal and reproductive systems3 and more studies recently are reported for chronic diabetes4 and osteoarthritis.5 One of the major zinc role is for the growth, development and maintenance of bone. Conversely, bone growth retardation is a common finding in various conditions associated with zinc deficiency.6789101112 For bone formation, zinc has been known to promote bone formation in osteoblasts and inhibits bone resorption in osteoclast.89 In particular, our previous studies demonstrated that zinc increased bone formation and mineralization by stimulating alkaline phosphatase (ALP) activity, the key enzyme for bone calcification and collagen type I (COL-1) for the extracellular matrix mineralization both in osteoblast and mice model.131415161718
Zinc has been shown to stimulate the expression of a particular transcription factor which is related to osteoblast differentiation, runt-related transcription factor 2 (Runx2).7 Runx2 has been widely accepted as the master osteogenic transcription factor and it plays a critical role in osteoblast marker gene expression.1920 As the BMP-2 signaling cascade is critically activated to osteoblast marker gene expression, the signaling cascade is involved in stimulating osteogenic transcription factor Runx2, osterix, and DLX5 expression.212223 Recently, a short-term Zn-deficient diet decreases bone formation through downregulating BMP-2 in rat bone.24 However, the modulation by zinc on BMP-2 signaling and bone formation in osteoblastic cells has not been fully determined.
Therefore, we investigated whether zinc modulated BMP-2 signaling pathway, thus affected Runx2 and osterix which upregulate the gene expression of bone marker proteins in osteoblastic MC3T3-E1 cells. Furthermore, we also evaluated alkaline phosphatase (ALP) activity and collagen type I (COL-1), and osteoblast marker genes under zinc deficiency for bone formation by osteoblasts.
Methods
Reagents
MC3T3-E1 subclone 4 (SC4, high osteoblast differentiation, ATCC, CRL-2593) and 24 (SC24, lower osteoblast differentiation, ATCC, CRL-2594) were obtained from ATCC Cell Bank (Manassas, VA, USA). Cell culture reagents and TRIzol reagent were obtained from Gibco Laboratories and Invitrogen both (Grand Island, NY, USA). Antibodies for target proteins, BMP-2, Smad-1, P-Smad-1, Runx2, osterix, alkaline phosphatase (ALP), and Pro COL-1 were obtained from Santa Cruz (Santa Cruz, CA, USA). Chemicals were obtained from Sigma (St Louis, MO, USA). All other plastic wares used were trace element free analysis grade.
Cell culture and zinc treatment
To test zinc effect on osteoblasts, we used both low and high osteoblast differentiation cell lines: MC3T3-E1 subclone 24 (SC 24, lower osteoblast differentiation) and subclone 4 (SC 4, high osteoblast differentiation). Cells were cultured in regular growth media (seeding density, 1 × 105 cells/mL, α-MEM with 10% FBS, 1 mM sodium pyruvate and 1% penicillin and streptomycin) in a humidified atmosphere (5% CO2 at 37℃) up to 80% confluence. Then, the cells were cultured with the osteogenic differentiation media with zinc (as ZnCl2) as Zn-deficient (Zn−, 1 µM) or Zn-adequate (Zn+, 15 µM). The normal osteogenic differentiation media (OSM) was used as normal control (growth media plus 3 mM glycerol 2-phosphate and 50 µg/mL ascorbic acid for osteoblast differentiation). For zinc treatment, cellular zinc chelator TPEN (cell-permeable zinc metal chelator, 5 µM) was added with each designated zinc level media to induce the cellular zinc depletion.
Cell viability
Cell viability was assessed by MTT assay based on metabolic reduction 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) in living cells. Cells (1 × 104 cells/well in 96-well plate) were plated in growth media for 24 h at 5% CO2, 37℃ and after then cells were cultured with Zn (1 or 15 µM) for another 24 h. The reduction of MTT in cells were measured followed by the commercial instructions (Abcam, Cambridge, UK). The reduction of MTT of cell culture was read on Micro Elisa reader (TECAN, Austria) at 570 nm.
Alkaline phosphatase (ALP) activity assay
ALP activity was measured by converting p-nitrophenyl phosphate (pNPP) as substrate to p-Nitrophenol (PNP) as product as previous described.14 Cellular protein was measured by the Bradford method. ALP activity in cells and media are expressed as nmol PNP/mg protein/min and nmol PNP/mL/min respectively as unit of enzyme activity.
Collagen and BMP-2 Measurement
The collagen synthesis by cells and the secretion outside cells to media were measured as previous described using cellular extracts and cell culture supernatnants as followed by commercial instructions.14 Cellular BMP-2 secretion was meausred by measuring BMP-2 concentration in cell medium supernatant using commertial BMP-2 ELISA kit (Abcam, Cambridge, UK).
Real-time PCR analysis
Transcription level (mRNA) for ALP, COL-1, osteocalcin (OC), osteopontin (OPN), Runx2, osterix (OX) and BMP-2 were measured using quantitative real-time PCR (qRT-PCT). To increase the yield of RNA, total RNA was extracted using Trizol (Thermo) with an overnight precipitation. For real-time analysis, cDNA was transcribed from a total of 600 ng of DNase I-treated RNA using the cDNA reverse-transcription kit and random primers (Thermo). Quantitative Real-time reverse-transcriptase polymerase chain reaction (qRT-PCR) was performed using a Mx3000p System (Stratagene). To determine relative mRNA expression, housekeeping gene (β-actin) and cell apoptosis marker gene with SYBR green I (SYBR Advantage qPCR Premix) were used.
Immunoblot analysis
Protein expression (ALP, COL-1, Runx2, osterix, BMP-2, Smad-1 and p-Smad-1) was measured using Western blotting, as described previously.14 The primary antibodies for target proteins were purchased from Bio-Rad and Abcam. Briefly, cells were lysed with RIPA buffer, and the cellular and the nuclear proteins were extracted. Protein concentration was determined using the BCA Protein Assay Kit, and the equal amount of protein from each sample was separated by SDS/PAGE and transferred to nitrocellulose membranes. After blocking with 5% skim milk in PBS-T (PBS/0.1% Tween-20) and washed with PBST and then incubated with primary antibodies (target proteins, Bio-Rad, Abcam) for overnight at 4℃. After that, the blot was incubated with the secondary antibodies. Relative protein images were determined by using HRP-conjugated secondary antibodies and ECL substrates (Thermo-Fishers).
Statistical analysis
Data were analyzed using SPSS program (version 17). Mean difference was considered the significance as p < 0.05. Mean comparison was performed by one way ANOVA to test the effect of different zinc levels (OSM, Zn− and Zn+). Once significance was detected, then Turkey's HSD test as post-hoc test was used for the comparison of difference between groups.
Results
Zinc deficiency decreased cellular and extracellular ALP activity and collagen synthesis in MC3T3-E1 cells
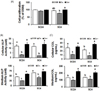
Cell viability was measured to determine cell death by zinc deficiency under the condition of present study. In high osteoblast differentiation cell type (SC 4), cell viability in Zn− was lower than Zn+, however it was not different with normal control OSM. This confirms no severe effect for cell viability by zinc deficiency level for the present study (Fig. 1A).
ALP activity produces inorganic phosphate and collagen synthesis is necessary which both events are the major actions for bone formation in osteoblast differentiation and extracellular matrix mineralization.25 We measured ALP activity that both cellular (intracellular) and medium (extracellular), and ALP activity decreased by zinc deficiency in both low (SC 24) and high (SC 4) osteoblast differentiation cell lines (Fig. 1B). Cellular collagen-I concentration (within cells) was low in Zn− and the patterns was similar to the pattern of ALP activity by zinc, while medium collagen-I concentration (secreted outside cells, extracellular) was less affected by zinc deficiency, compared to OSM (Fig. 1C). The results indicated that zinc deficiency in osteoblasts decreased ALP activity and collagen synthesis both in intracellular (synthesized within cells) and extracellular matrix (secreted outside cells).
BMP-2 expression and the activation of Smad-1 were decreased by zinc deficiency in MC3T3-E1 cell
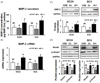
We first examined whether cellular zinc deficiency in MC3T3-E1 cells affected BMP-2 secretion. Zinc deficiency decreased medium BMP-2 level (the secreted BMP-2 outside cells) in both subclone 4 and 24 lines (Fig. 2A), and this suggests zinc modulates BMP-2 signaling in osteoblastic MC3T3-E1 cells. Next, we examined whether this decreased BMP-2 secretion, which was due to zinc deficiency, modulated BMP-2 signaling moledules. BMP-2 mRNA and protein expression were downregulated under Zn−, as compared to OSM or Zn+ (Fig. 2B, C).
We further tested whether zinc deficiency in osteoblasts affected Smad-1 expression and the activation of Smad-1, a BMP-2 downstream regulator. The activation of Smad-1 upregulates the expression of bone-specific transcription factors, such as Runx2 and osterix. The results show that Smad-1 and the phosphorylated-Smad-1 (p-Smad-1) protein expression was downregulated by zinc deficiency in osteoblasts (Fig. 2D). This implies that zinc deficiency in MC3T3-E1 cells inhibits BMP-2-induced Smad-1 activation (phosphorylated Smad-1), too.
Zinc deficiency downregulated the expression of bone-specific transcription factors (Runx2 and osteorix) in osteoblasts
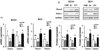
Both Runx2 and osterix are osteoblastic differentiation transcription factors as well as downstream molecules in BMP-2 signaling. These two transcription factors modulate the transcription of osteoblast differentiation marker genes. By zinc deficiency (Zn−) in osteoblast, both gene and protein expression of Runx2 were down-regulated, as osterix was too, in both high (SC24) and low (SC4) osteogenic cell lines (Fig. 3A, B).
Zinc deficiency down-regulated the gene expression of bone marker proteins (ALP, COL-1) as well as osteoblast differentiation genes (osteocalcin, osteopontin)
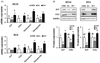
We investigated whether zinc modulates the bone-related gene expression (alkaline phosphatase, ALP; collagen type I, COL-1; osteocalcin; osteopontin) in both low (SC 24) and high (SC 4) osteogenic cell lines. Our data showed that transcription of ALP and COL I was prominently downregulated by Zn−, as compared to OSM and Zn+, both in low and high osteogenic cell lines (Fig. 4A). Two osteoblast differentiation genes, osteocalcin and osteopontin, gene expression of two genes were downregulated by zinc deficiency which was the same pattern of ALP and COL I too. We also observed that zinc deficiency (Zn−) downregulated protein expression ALP and COL-I, compared to OSM and Zn+, both cell types (Fig. 4B). These results showed that zinc deficiency in osteoblasts downregulated the expression of bone-related marker protein (ALP, COL-I) as well as osteoblast differentiation markers (osteocalcin, osteopontin).
Discussion
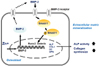
Zinc is well known to stimulate bone formation and bone matrix mineralization. Our and others' studies reported that zinc deficiency causes the decreased osteoblast differentiation and growth retardation in both in vitro and in vivo models.13141516171819 In this study, we determine in osteoblasts that, 1) zinc deficiency decreases BMP-2 signaling by downregulating BMP gene and protein expression and synthesis, and furthermore 2) downregulating BMP-2 downstream regulator Smad-1 and its phosphorylated p-Smad-1. 3) This decreased Smad-1 activation to p-Smad-1 by zinc deficiency in osteoblasts then can downregulates bone-specific transcription factors Runx2 and osterix expression, and 4) furthermore this decreased Runx2 and osterix expression can cause the decrease of bone marker and osteoblast differentiation marker protein synthesis and enzyme activity (ALP, collagen I, osteocalcin and osteopontin). Therefore it can be concluded from the findings of the present study that inadequate zinc level in osteoblasts can cause poor osteoblast differentiation by downregulating BMP-2 signaling via downregulating BMP-2 synthesis and by down-activating the downstream regulator Smad-1 (Fig. 5).
BMP-2, members of the TGF-β superfamily, is the main growth factor that promotes the differentiation of mesenchymal cells into osteoblasts or chondroblast.2223 This BMP-2 induces Runx2/Cbfa1 mRNA in C2C12 myoblast and this cooperation was induced by the expression of Runx2 and Smad1/5.21 Recent report demonstrated that BMP-2 mRNA expression was decreased in rat bone by zinc deficiency.24 Indeed, we here found that the expression and activation of BMP-2/Smad-1 signaling molecules were down regulated by zinc deficiency. Our data showed that the expressions of BMP-2 (mRNA and protein) were downregulated by zinc deficiency in osteoblasts. Smad-1 and its activated p-Smad-1, the downstream regulators in BMP-2 signaling proteins, were also downregulated by zinc deficiency. BMP-2 target genes are transcription factors that have essential roles in skeletal development, including Runx2 and osterix.23 Our data demonstrated that even in short-term zinc deficiency decreased BMP-2 as well as Runx2 and osterix expression. Furthermore, this downregulated expression of bone-specific transcription factors, Runx2 and osterix, can cause the down-regulation of osteoblast differentiation marker gene and protein expression.
Bone-specific transcription factors, Runx2 and osterix, are found to be essential molecules for osteoblast differentiation as indicated by the fact that both Runx2-null mice and osterix-null mice have neither bone tissue nor osteoblasts.26 As BMP-2 signaling downstream regulator protein, Runx2 is an essential osteoblastic differentiation transcription factor.27 The mRNA and protein expression of Runx2 and osterix were significantly downregulated by zinc deficiency. Our data also showed that the expression of bone marker and osteoblast differentiation genes (ALP, COL-1, osteocalcin and osteopontin) were significantly suppressed by zinc deficiency.
Two proteins, alkaline phosphatase (ALP) and collagen 1, are related to extracellular matrix mineralization in osteoblasts and both proteins are the major components in hard tissue formation.252829 Collagen 1 is the most abundant collagenous bone matrix protein and it is synthesized by and secreted outside osteoblasts.25 Zinc in the bone tissues may first cause the activation of alkaline phosphatase and the stimulation of collagen synthesis in osteoblasts, which are involved in bone mineralization and calcification.30 In our short-term (1 week) zinc deficiency in osteoblasts decreased the expression of BMP-2 and osteoblast differentiation marker genes. It is also reported that BMP-2-induced Runx2 expression is mediated by Dlx5.21 Also in this study, we used two types of osteoblast cell lines which are high (SC4) and low (SC24) osteoblast differentiation, depending on osteoblast differentiation levels, and this would be useful for interpreting the data for the cases for normal and poor osteoblast differentiation levels in human subjects as appropriate.
In conclusion, this study showed that zinc deficiency in osteoblasts causes down-activating BMP-2 signaling molecules via downregulating BMP-2 and Smad-1/Smad-1 activation and this causes the downregulation of two key bone transcription factors Runx2 and osterix, and finally suppressing bone marker gene and protein expression. The study results suggest that adequate zinc supplementation may prevent poor bone formation by blocking zinc deficiency-induced downactivating BMP-2 signaling and thus by preventing poor osteoblast differentiation.
Summary
This study was aimed to evaluate whether cellular zinc inadequacy modulated BMP-2 signaling which affected the expression of bone-specific transcription factors (Runx2 and osterix) and bone marker gene and protein expression, therefore finally affected osteoblast differentiation. Using osteoblast model (mouse origin MC3T3-E1 cell lines), we found that zinc deficiency downactivaed BMP-2 signaling by downregulating BMP-2 expression and its downstream regulator Smad-1 and its activation (phosphorylated Smad-1). These downregulated BMP-2 and Smad-1/p-Smad-1 consecutively downregulated two bone-specific transcription factors, Runx2 and osterix, and furthermore it caused the decrease of bone marker gene and protein (ALP, COL-1, osteocalcin and osteopontin) expression and synthesis. All these findings from the present study imply that inadequate zinc status in osteoblasts can cause poor osteoblast differentiation by decreasing BMP-2 signaling by downregulating BMP-2 synthesis and its downstream regulator Smad-1 activation. Runx2 would be the key regulator to be poorly activated transcription factor by the down-activated BMP-2 signaling by zinc deficiency in osteoblasts.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download