Abstract
Purpose
Many studies have suggested that neuronal cells protect against oxidative stress-induced apoptotic cell death by polyphenolic compounds. We investigated the neuroprotective effects and the mechanism of action of Momordica charantia ethanol extract (MCE) against H2O2-induced cell death of human neuroblastoma SK-N-MC cells.
Methods
The antioxidant activity of MCE was measured by the quantity of total phenolic acid compounds (TPC), quantity of total flavonoid compounds (TFC), and 2,2-Diphenyl-1-pycrylhydrazyl (DPPH) radical scavenging activity. Cytotoxicity and cell viability were determined by CCK-8 assay. The formation of reactive oxygen species (ROS) was measured using 2,7-dichlorofluorescein diacetate (DCF-DA) assay. Antioxidant enzyme (SOD-1,2 and GPx-1) expression was determined by real-time PCR. Mitogen-activated protein kinases (MAPK) pathway and apoptosis signal expression was measured by Western blotting.
Results
The TPC and TFC quantities of MCE were 28.51 mg gallic acid equivalents/extract g and 3.95 mg catechin equivalents/extract g, respectively. The IC50 value for DPPH radical scavenging activity was 506.95 µg/ml for MCE. Pre-treatment with MCE showed protective effects against H2O2-induced cell death and inhibited ROS generation by oxidative stress. SOD-1,2 and GPx-1 mRNA expression was recovered by pre-treatment with MCE compared with the presence of H2O2. Pre-treatment with MCE inhibited phosphorylation of p38 and the JNK pathway and down-regulated cleaved caspase-3 and cleaved PARP by H2O2.
Figures and Tables
Fig. 1
Protective effects of Momordica charantia ethanol extracts (MCE) on H2O2-induced cell death and cytotoxicity in SK-N-MC cells. (A) Cell viability was determined by CCK-8 assay. SK-N-MC cell wre treated with increasing concentrations (100~600 µM) of H2O2 500 µM for 24 hr. (B) SK-N-MC cell were treated with increasing concentrations (1~50 µg/ml) of MCE for 24 hr. (C) The cell pretreated with MCE at the indicated concentration (1~50 µg/ml) for 6hr, and then treated with H2O2 for 18 hr. Value represent the mean ± SD. *p < 0.05, **p < 0.01 compared with control. #p < 0.05 vs compared with H2O2 treated cell

Fig. 2
Intracellular ROS scavenging activity of MCE. Cells were treated with H2O2 and/ or MCE as described in the legend of Fig. 1C. The generation of intracellular ROS was determined by DCF-DA methods using a fluorescence spectrophotometer with excitation and emission wavelengths of 485 nm and 530 nm, respectively. Value represent the mean ± SD. Means with different letters (a~c) at each mRNA are significantly different (p < 0.05) by Duncan's multiple range test.

Fig. 3
Effects of MCE on H2O2-induced mRNA expression levels of antioxidant enzymes in SK-N-MC cells. Cells were treated with H2O2 and/ or MCE as described in the legend of Fig. 1C. The mRNA levels of antioxidant enzymes were determined by real-time PCR analysis. (A) SOD-1, superoxide dismutase-1; (B) SOD-2, superoxide dismutase-2; (C) GPx1, glutathione peroxidase-1. Value represent the mean ± SD. Means with different letters (a~d) at each mRNA are significantly different (p < 0.05) by Duncan's multiple range test.
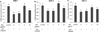
Fig. 4
Effects of MCE on H2O2-induced phosphorylation of MAPK (p38 and JNK). Pathway in SK-N-MC cells. Cells were treated with H2O2 and/ or MCE as described in the legend of Fig. 1C. The protein levels of MAPKs pathway signals were analyzed by western blotting, and normalizes to the GAPDH level. The density of each protein band was quantified by using Bio-1D imaging software (Vilber Lourmat, Marne-la-Vallée, France). Value represent the mean ± SD. Means with different letters (a~d) at each mRNA are significantly different (p < 0.05) by Duncan's multiple range test.
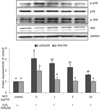
Fig. 5
MCE attenuates H2O2-induced cleavage of caspase-3 and PARP in SK-N-MC cells. Cells were treated with H2O2 and/ or MCE as described in the legend of Fig. 1C. The protein levels of caspase-3, cleaved caspase-3 (A), PARP and cleaved PARP (B) were analyzed by western blotting, and normalizes to the GAPDH level. Value represent the mean ± SD. Means with different letters (a~e) at each mRNA are significantly different (p < 0.05) by Duncan's multiple range test.
References
1. Statistics Korea. Korean social trends 2016. Daejeon: Statistics Korea;2016.
2. Kim HB. Current Status and Implications of disease studies related to aging. Seoul: Korea Institute of Science & Technology Evaluation and Planning;2012.
3. Budzynska B, Boguszewska-Czubara A, Kruk-Slomka M, Skalicka-Wozniak K, Michalak A, Musik I, Biala G. Effects of imperatorin on scopolamine-induced cognitive impairment and oxidative stress in mice. Psychopharmacology (Berl). 2015; 232(5):931–942.

4. Hou CW, Chang SY, Jeng KC. Protective effect of a sesamin derivative, 3-bis (3-methoxybenzyl) butane-1, 4-diol on Abeta-stressed PC12 cells. Arch Pharm Res. 2015; 38(4):543–548.
5. Murakami S, Miyazaki I, Sogawa N, Miyoshi K, Asanuma M. Neuroprotective effects of metallothionein against rotenoneinduced myenteric neurodegeneration in parkinsonian mice. Neurotox Res. 2014; 26(3):285–298.

6. Kang SW. Role of reactive oxygen species in cell death pathways. Hanyang Med Rev. 2013; 33(2):77–82.

7. Stadtman ER, Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Chem Res Toxicol. 1997; 10(5):485–494.

8. Heo HJ, Lee CY. Strawberry and its anthocyanins reduce oxidative stress-induced apoptosis in PC12 cells. J Agric Food Chem. 2005; 53(6):1984–1989.

9. Kim D, Chae HS, Kim NY, Jang A. Anti-oxidative activity and the protective effect of donkey's bone and skin extracts on SK-N-SH cell. J Life Sci. 2013; 23(8):1019–1024.
10. Kwon KH, Lim H, Chung MJ. Neuroprotective effects of bread containing Cirsium setidens or Aster scaber. J Korean Soc Food Sci Nutr. 2014; 43(6):829–835.

11. Chung MJ, Park YI, Kwon KH. Neuroprotective effects of cirsium setidens, pleurospermum kamtschaticumin, and allium victorials based on antioxidant and p38 phosphorylation inhibitory activities in SK-N-SH neuronal cells. J Korean Soc Food Sci Nutr. 2015; 44(3):347–355.

12. Kim SH, Choi HJ, Oh HT, Chung MJ, Cui CB, Ham SS. Cytoprotective effect by antioxidant activity of codonopsis lanceolata and platycodon grandiflorum ethyl acetate fraction in human HepG2 cells. Korean J Food Sci Technol. 2008; 40(6):696–701.
13. Kim SM, Chung MJ, Ha TJ, Choi HN, Jang SJ, Kim SO, Chun MH, Do SI, Choo YK, Park YI. Neuroprotective effects of black soybean anthocyanins via inactivation of ASK1-JNK/p38 pathways and mobilization of cellular sialic acids. Life Sci. 2012; 90(21-22):874–882.

14. Choi HN, Chung MJ, Park JK, Park YI. Neuroprotective effects of N-acetylglucosamine against hydrogen peroxide-induced apoptosis in human neuronal SK-N-SH cells by inhibiting the activation of caspase-3, PARP, and p38. Food Sci Biotechnol. 2013; 22(3):853–858.

15. Chen B, Yue R, Yang Y, Zeng H, Chang W, Gao N, Yuan X, Zhang W, Shan L. Protective effects of (E)-2-(1-hydroxyl-4-oxocyclohexyl) ethyl caffeine against hydrogen peroxide-induced injury in PC12 cells. Neurochem Res. 2015; 40(3):531–541.

16. Garcimartín A, Merino JJ, González MP, Sánchez-Reus MI, Sánchez-Muniz FJ, Bastida S, Benedí J. Organic silicon protects human neuroblastoma SH-SY5Y cells against hydrogen peroxide effects. BMC Complement Altern Med. 2014; 14(1):384–392.

17. Tian X, Guo LP, Hu XL, Huang J, Fan YH, Ren TS, Zhao QC. Protective effects of Arctium lappa L. roots against hydrogen peroxide-induced cell injury and potential mechanisms in SH-SY5Y cells. Cell Mol Neurobiol. 2015; 35(3):335–344.

18. Araba BG. Stimulation of protein biosynthesis in rat hepatocytes by extracts of Momordica charantia. Phytother Res. 2001; 15(2):95–98.
19. Grover JK, Yadav SP. Pharmacological actions and potential uses of Momordica charantia: a review. J Ethnopharmacol. 2004; 93(1):123–132.

20. Schmourlo G, Mendonça-Filho RR, Alviano CS, Costa SS. Screening of antifungal agents using ethanol precipitation and bioautography of medicinal and food plants. J Ethnopharmacol. 2005; 96(3):563–568.

21. Lee HJ, Moon JH, Lee WM, Lee SG, Kim AK, Woo YH, Park DK. Charantin contents and fruit characteristics of bitter gourd (Momordica charantia L.) accessions. J Bio Environ Control. 2012; 21(4):379–384.

22. Divya D, Hettiarachchy NS, Ganesh V, Kannan A, Rayaprolu S. Phenolic extracts from leaves of bitter melon (Momordica charantia) with antioxidant properties. J Agric Sci Appl. 2013; 2(1):28–34.

23. Tan SP, Kha TC, Parks SE, Roach PD. Bitter melon (Momordica charantia L.) bioactive composition and health benefits: a review. Food Rev Int. 2016; 32(2):181–202.
24. Kim HW, Shin H, Hwang D, Lee J, Jeong H, Kim D. Functional cosmetic characteristics of momordica charantia fruit extract. Korean Chem Eng Res. 2015; 53(3):289–294.

25. Lee YR. Nutritional components and antioxidant activity of dry bitter melon (Momordica charantia L.). J Korean Soc Food Sci Nutr. 2016; 45(4):518–523.

26. Sin SM, Mok SY, Lee S, Cho KM, Cho EJ, Kim HY. Protective effect of bitter melon (Momordica charantia) against oxidative stress. Cancer Prev Res. 2011; 16(1):86–92.
27. Choi JR, Choi JM, Lee SH, Cho KM, Cho EJ, Kim HY. The protective effects of protocatechuic acid from momordica charantia against oxidative stress in neuronal cells. Korean J Pharmacogn. 2014; 45(1):11–16.
28. Gong J, Sun F, Li Y, Zhou X, Duan Z, Duan F, Zhao L, Chen H, Qi S, Shen J. Momordica charantia polysaccharides could protect against cerebral ischemia/reperfusion injury through inhibiting oxidative stress mediated c-Jun N-terminal kinase 3 signaling pathway. Neuropharmacology. 2015; 91:123–134.

29. Duan ZZ, Zhou XL, Li YH, Zhang F, Li FY, Su-Hua Q. Protection of Momordica charantia polysaccharide against intracerebral hemorrhage-induced brain injury through JNK3 signaling pathway. J Recept Signal Transduct Res. 2015; 35(6):523–529.
30. Lee KH, Lee SJ, Lee HJ, Choi GE, Jung YH, Kim DI, Gabr AA, Ryu JM, Han HJ. Amyloid β1-42 (Aβ1-42) induces the CDK2-mediated phosphorylation of tau through the activation of the mTORC1 signaling pathway while promoting neuronal cell death. Front Mol Neurosci. 2017; 10:229.

31. Lee HJ, Ryu JM, Jung YH, Lee SJ, Kim JY, Lee SH, Hwang IK, Seong JK, Han HJ. High glucose upregulates BACE1-mediated Aβ production through ROS-dependent HIF-1α and LXRα/ABCA1-regulated lipid raft reorganization in SK-N-MC cells. Sci Rep. 2016; 6:36746.

32. Boo HO, Lee HH, Lee JW, Hwang SJ, Park SU. Different of total phenolics and flavonoids, radical scavenging activities and nitrite scavenging effects of momordica Charantia L. according to cultivars. Korean J Med Crop Sci. 2009; 17(1):15–20.
33. Hossain H, Shahid-Ud-Daula AF, Jahan IA, Nimmi I, Hasan K, Haq MM. Evaluation of antinociceptive and antioxidant potential from the leaves of spilanthes paniculata growing in Bangladesh. Int J Pharm Phytopharm Res. 2012; 1(4):178–186.
34. Babu D, Gurumurthy P, Borra SK, Cherian KM. Antioxidant and free radical scavenging activity of triphala determined by using different in vitro models. J Med Plant Res. 2013; 7(39):2898–2905.
35. Park JS, Kim HS, Chin KB. The antioxidant activity of Yacon (Polymnia sonchifoliaty) and its application to the pork patties as a natural antioxidant. J Korean Soc Food Sci Anim Resour. 2012; 32(2):190–197.

36. Lee JH, Chin KB. Evaluation of antioxidant activities of red beet extracts, and physicochemical and microbial changes of ground pork patties containing red beet extracts during refrigerated storage. J Korean Soc Food Sci Anim Resour. 2012; 32(4):497–503.

37. Yoo HG, Lee BH, Kim W, Lee JS, Kim GH, Chun OK, Koo SI, Kim DO. Lithospermum erythrorhizon extract protects keratinocytes and fibroblasts against oxidative stress. J Med Food. 2014; 17(11):1189–1196.

38. Kim EJ, Choi JY, Yu MR, Kim MY, Lee SH, Lee BH. Total polyphenols, total flavonoid contents, and antioxidant activity of Korean natural and medicinal plants. Korean J Food Sci Technol. 2012; 44(3):337–342.

39. Choi DJ, Kim SL, Choi JW, Park YI. Neuroprotective effects of corn silk maysin via inhibition of H2O2-induced apoptotic cell death in SK-N-MC cells. Life Sci. 2014; 109(1):57–64.
40. Chung MJ, Lee S, Park YI, Lee J, Kwon KH. Neuroprotective effects of phytosterols and flavonoids from Cirsium setidens and Aster scaber in human brain neuroblastoma SK-N-SH cells. Life Sci. 2016; 148:173–182.

41. Davies KJ. The broad spectrum of responses to oxidants in proliferating cells: a new paradigm for oxidative stress. IUBMB Life. 1999; 48(1):41–47.

42. Saladino AJ, Willey JC, Lechner JF, Grafstrom RC, LaVeck M, Harris CC. Effects of formaldehyde, acetaldehyde, benzoyl peroxide, and hydrogen peroxide on cultured normal human bronchial epithelial cells. Cancer Res. 1985; 45(6):2522–2526.
43. Kim AK, Kim JH. Alterations of antioxidant enzymes in respones to oxidative stress and antioxidants. J Appl Pharmacol. 2001; 9(4):249–257.
44. Pan J, Chang Q, Wang X, Son Y, Zhang Z, Chen G, Luo J, Bi Y, Chen F, Shi X. Reactive oxygen species-activated Akt/ASK1/p38 signaling pathway in nickel compound-induced apoptosis in BEAS 2B cells. Chem Res Toxicol. 2010; 23(3):568–577.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download