Abstract
Purpose
Neuroinflammation is mediated by activation of microglia implicated in the pathogenesis of neurodegenerative disorders such as Alzheimer's disease and Parkinson's disease. Inhibition of neuroinflammation may be an effective solution to treat these brain disorders. Petalonia binghamiae is known as a traditional food, based on multiple biological activities such as anti-oxidant and anti-obesity. In present study, the anti-neuroinflammatory potential of Petalonia binghamiae was investigated in LPS-stimulated BV2 microglial cells. Methods: Cell viability was measured by MTT assay. Production of nitric oxide (NO) was examined using Griess reagent. Expression of inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2) was detected by Western blot analysis. Activation of nuclear factor κB (NF-κB) signaling was examined by nuclear translocation of NF-κB p65 subunit and phosphorylation of IκB. Results: Extract of Petalonia binghamiae significantly inhibited LPS-stimulated NO production and iNOS/COX-2 protein expression in a dose-dependent manner without cytotoxicity. Pretreatment with Petalonia binghamiae suppressed LPS-induced NF-κB p65 nuclear translocation and phosphorylation of IκB. Co-treatment with Petalonia binghamiae and pyrrolidine duthiocarbamate (PDTC), an NF-κB inhibitor, reduced LPS-stimulated NO release compared to that in PB-treated or PDTC-treated cells. Conclusion: The present results indicate that extract of Petalonia binghamiae exerts anti-neuroinflammation activities, partly through inhibition of NF-κB signaling. These findings suggest that Petalonia binghamiae might have therapeutic potential in relation to neuroinflammation and neurodegenerative diseases.
Go to : 
References
1. Nakagawa Y, Chiba K. Role of microglial m1/m2 polarization in relapse and remission of psychiatric disorders and diseases. Pharmaceuticals (Basel). 2014; 7(12):1028–1048.

2. Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010; 87(5):779–789.

4. Chung YC, Ko HW, Bok E, Park ES, Huh SH, Nam JH, Jin BK. The role of neuroinflammation on the pathogenesis of Parkinson's disease. BMB Rep. 2010; 43(4):225–232.

5. González H, Elgueta D, Montoya A, Pacheco R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. J Neuroimmunol. 2014; 274(1–2):1–13.

6. Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009; 108(6):1343–1359.

7. Yoon CH, Kim DC, KO WM, Kim KS, Lee DS, Kim DS, Cho HK, Seo J, Kim SY, Oh H, Kim YC. Anti-neuroinflammatory effects of Quercetin-3-O-glucuronide isolated from the leaf of Vitis labrus-cana on LPS-induced neuroinflammation in BV2 cells. Korean J Pharmacogn. 2014; 45(1):17–22.
8. Dilshara MG, Jayasooriya RG, Lee S, Choi YH, Kim GY. Morin downregulates nitric oxide and prostaglandin E2 production in LPS-stimulated BV2 microglial cells by suppressing NF-κB activity and activating HO-1 induction. Environ Toxicol Pharmacol. 2016; 44:62–68.

9. Park E, Chun HS. Green tea polyphenol Epigallocatechine gallate (EGCG) prevented LPS-induced BV-2 micoglial cell activation. J Life Sci. 2016; 26(6):640–645.

10. Min JS, Lee DS. A screen for dual-protection molecules from a natural product library against neuronal cell death and microglial cell activation. J Life Sci. 2015; 25(6):656–662.

11. Galea E, Reis DJ, Fox ES, Xu H, Feinstein DL. CD14 mediate endotoxin induction of nitric oxide synthase in cultured brain glial cells. J Neuroimmunol. 1996; 64(1):19–28.
12. Laflamme N, Rivest S. Toll-like receptor 4: the missing link of the cerebral innate immune response triggered by circulating gramnegative bacterial cell wall components. FASEB J. 2001; 15(1):155–163.

13. Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009; 4(4):399–418.

14. Lee YP, Kang SY. A catalogue of the seaweeds in Korea. Jeju: Jeju National University Press;2002.
15. Noda H, Amano H, Arashima K, Hashimoto S, Nisizawa K. Studies on the antitumour activity of marine algae. Bull Jpn Soc Sci Fish. 1989; 55(7):1259–1264.

16. Schwartsmann G, Brondani da Rocha A, Berlinck RG, Jimeno J. Marine organisms as a source of new anticancer agents. Lancet Oncol. 2001; 2(4):221–225.

17. Shin DB, Han EH, Park SS. Cytoprotective effects of Phaeophyta extracts from the coast of Jeju island in HT-22 mouse neuronal cells. J Korean Soc Food Sci Nutr. 2014; 43(2):224–230.

18. Athukorala Y, Kim KN, Jeon YJ. Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem Toxicol. 2006; 44(7):1065–1074.

19. Ryu G, Park SH, Kim ES, Choi BW, Ryu SY, Lee BH. Cholinesterase inhibitory activity of two farnesylacetone derivatives from the brown alga Sargassum sagamianum. Arch Pharm Res. 2003; 26(10):796–799.
20. Park JC, Choi JS, Song SH, Choi MR, Kim KY, Choi JW. Hepatoprotective effect of extracts and phenolic compound from marine algae in bromobenzene-treated rats. Korean J Pharmacogn. 1997; 28(4):239–246.
21. Park KE, Jang MS, Lim CW, Kim YK, Seo Y, Park HY. Antioxidant activity on ethanol extract from boiled-water of Hizikia fusiformis. J Korean Soc Appl Biol Chem. 2005; 48(4):435–439.
22. Kang SI, Kim MH, Shin HS, Kim HM, Hong YS, Park JG, Ko HC, Lee NH, Chung WS, Kim SJ. A water-soluble extract of Petalonia binghamiae inhibits the expression of adipogenic regulators in 3T3-L1 preadipocytes and reduces adiposity and weight gain in rats fed a high-fat diet. J Nutr Biochem. 2010; 21(12):1251–1257.

23. Kang SI, Jin YJ, Ko HC, Choi SY, Hwang JH, Whang I, Kim MH, Shin HS, Jeong HB, Kim SJ. Petalonia improves glucose homeostasis in streptozotocin-induced diabetic mice. Biochem Biophys Res Commun. 2008; 373(2):265–269.

24. Yoon HS, Koh WB, Oh YS, Kim IJ. The Anti-melanogenic effects of Petalonia binghamiae extracts in α-melanocyte stimulating hormone-induced B16/F10 murine melanoma cells. J Korean Soc Appl Biol Chem. 2009; 52(5):564–567.

25. Lee SG, Kim MM. Anti-inflammatory effect of scopoletin in RAW264.7 macrophages. J Life Sci. 2015; 25(12):1377–1383.

26. Jiang Z, Li C, Arrick DM, Yang S, Baluna AE, Sun H. Role of nitric oxide synthases in early blood-brain barrier disruption following transient focal cerebral ischemia. PLoS One. 2014; 9(3):): e93134. e93134.

27. Wang JY, Lee CT, Wang JY. Nitric oxide plays a dual role in the oxidative injury of cultured rat microglia but not astroglia. Neuroscience. 2014; 281:164–177.

28. Blum-Degen D, Müller T, Kuhn W, Gerlach M, Przuntek H, Riederer P. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer's and de novo Parkinson's disease patients. Neurosci Lett. 1995; 202(1–2):17–20.
29. Li M, Dai FR, Du XP, Yang QD, Chen Y. Neuroprotection by silencing iNOS expression in a 6-OHDA model of Parkinson's disease. J Mol Neurosci. 2012; 48(1):225–233.

30. Gulati P, Singh N. Pharmacological evidence for connection of nitric oxide-mediated pathways in neuroprotective mechanism of ischemic postconditioning in mice. J Pharm Bioallied Sci. 2014; 6(4):233–240.
31. Okamoto S, Lipton SA. S-nitrosylation in neurogenesis and neuronal development. Biochim Biophys Acta. 2015; 1850(8):1588–1593.

32. Verstrepen L, Beyaert R. Receptor proximal kinases in NF-κB signaling as potential therapeutic targets in cancer and inflammation. Biochem Pharmacol. 2014; 92(4):519–529.

33. Carmody RJ, Chen YH. Nuclear factor-kappaB: activation and regulation during toll-like receptor signaling. Cell Mol Immunol. 2007; 4(1):31–41.
34. Hatziieremia S, Gray AI, Ferro VA, Paul A, Plevin R. The effects of cardamonin on lipopolysaccharide-induced inflammatory protein production and MAP kinase and NFkappaB signalling pathways in monocytes/macrophages. Br J Pharmacol. 2006; 149(2):188–198.
35. Gasparini C, Feldmann M. NF-κB as a target for modulating inflammatory responses. Curr Pharm Des. 2012; 18(35):5735–5745.
Go to : 
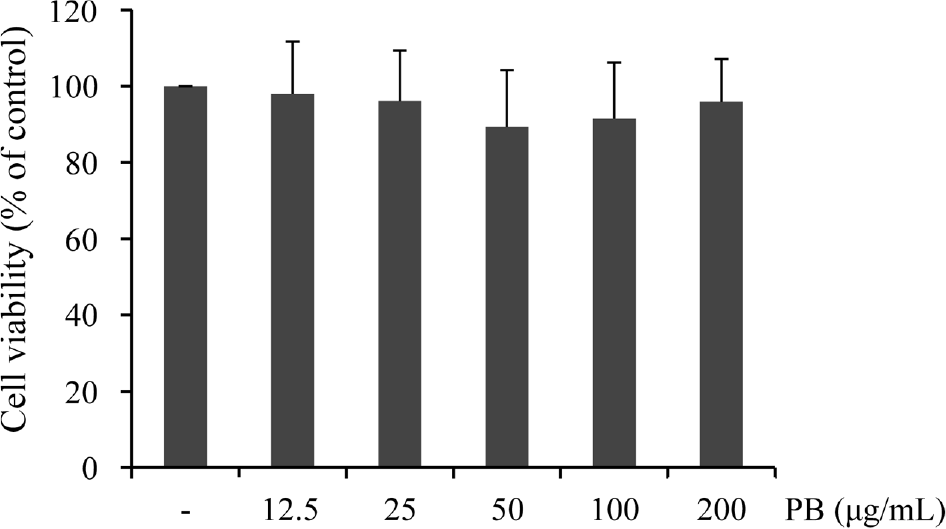 | Fig. 1.Effect of Petalonia binghamiae (PB) on the viability of BV-2 microglial cells. Cells were incubated with the indicated concentrations of PB for 24 h and cytotoxicity of PB was examined by MTT assay. Data are represented as mean ± SD of three independent experiments. |
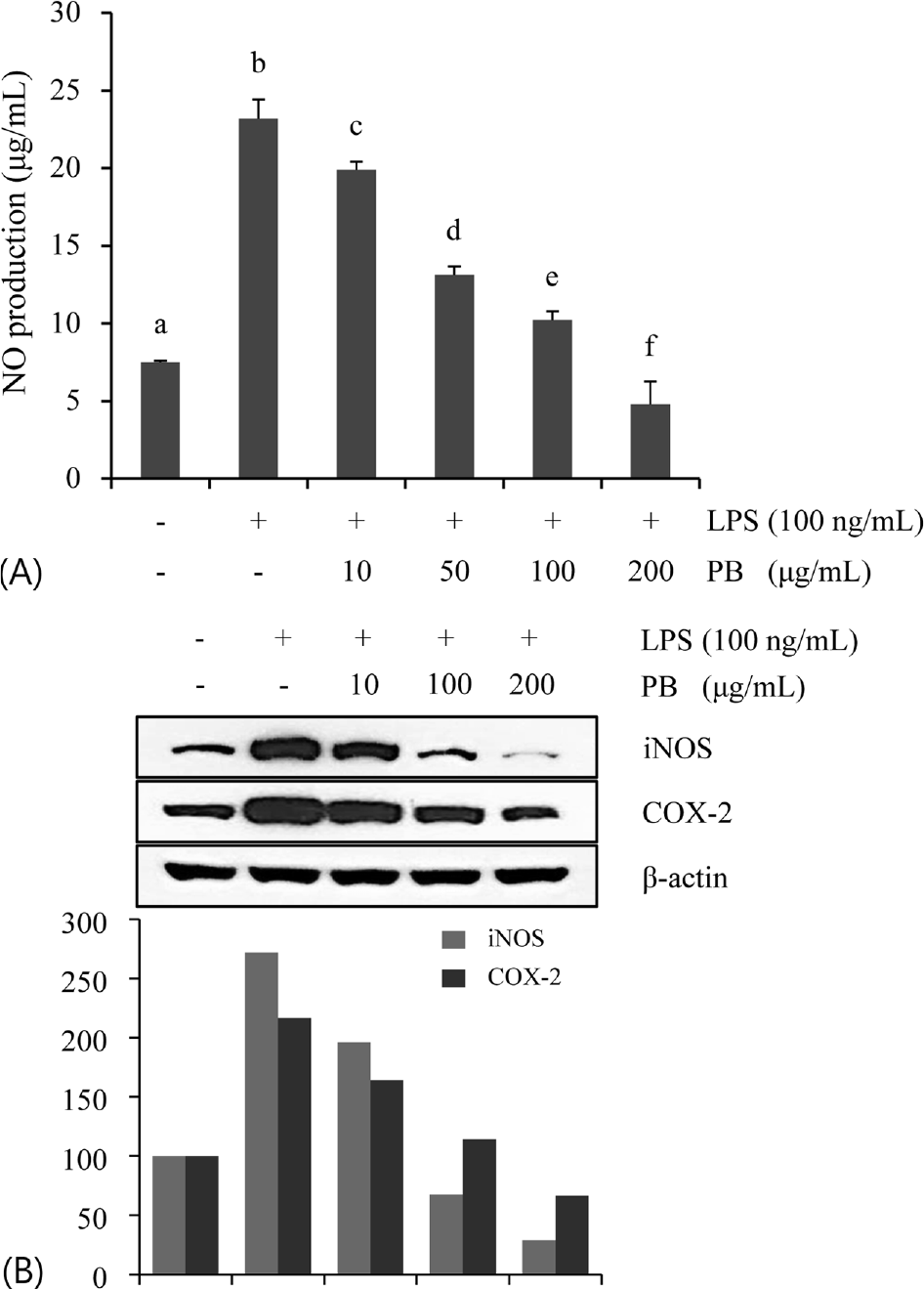 | Fig. 2.Inhibitory effect of Petalonia binghamiae (PB) on LPS-induced NO production (A) and iNOS/COX-2 protein expression (B). The cells were incubated with the indicated concentrations of PB for 30 min before treatment of LPS. Data are represented as mean±SD of three independent experiments. Means with different letter in superscript are significantly different (p < 0.05) by ANOVA and Duncan's multiple range test. |
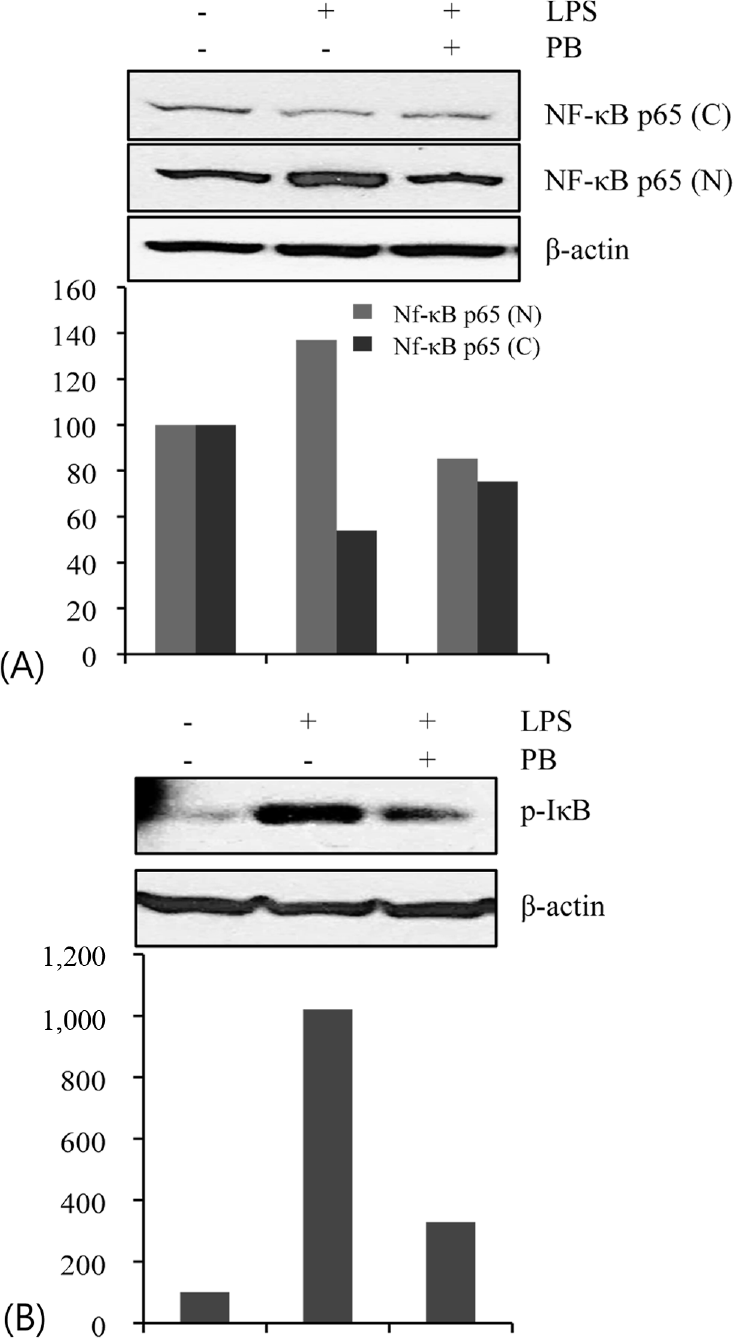 | Fig. 3.Effect of Petalonia binghamiae (PB) on the LPS-induced activation of NF-κB (p65) and IκB. The cells were treated with LPS (100 ng/mL) for 30 min in the absence and/or presence of PB (100 μg/mL). The cytoplasmic (C) and nuclear (N) extracts were prepared to determine translocation of NF-κB p65 and IκB activity was measured by levels of phosphorylated IκB protein. |
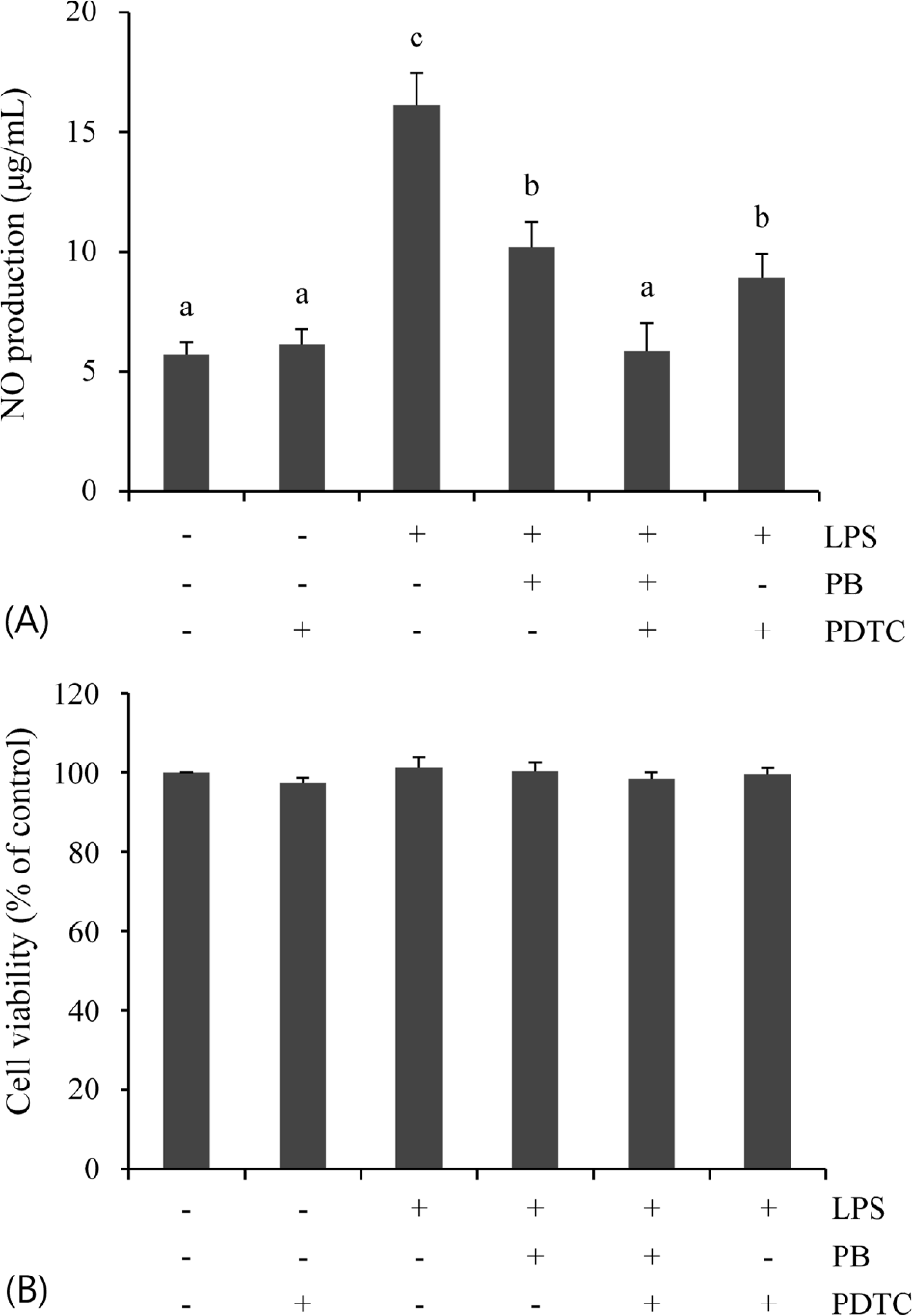 | Fig. 4.Involvement of NF-κB signaling on LPS-stimulated NO production. The cells were treated with LPS (100 ng/mL) for 24 h after treatment of PB (100 μg/mL) and/or PDTC (25 μM) for 30 min. Data are represented as mean ± SD of three independent experiments. Means sharing the same superscript letter are not are significantly different (p < 0.05) by ANOVA and Duncan's multiple range test. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download