Abstract
Purpose
Fruit and vegetable juices are known to be rich sources of antioxidants, which have beneficial effects on diseases caused by oxidative stress. The purpose of this study was to directly compare the antioxidant activities of fruit and vegetable juices marketed in Korea. Methods: We analyzed four fruit juices, two vegetable juices, two yellow-green juices, and six mixed vegetable juices. Antioxidant activities were analyzed using 2,2-diphenyl-1-picrylhydrazyl (DPPH) test, 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonate) (ABTS) test, and oxygen radical absorbance capacity (ORAC) assay. Protective effects against DNA damage were determined using an ex vivo comet assay with human lymphocytes. Results: DPPH radical scavenging activities were in the following order: blueberry juice > mixed vegetable C juice > kale juice > mixed vegetable P juice > grape juice. ABTS radical scavenging activities were in the following order: blueberry juice > mixed vegetable C juice > grape juice > mixed vegetable P juice > kale juice. Peroxyl radical scavenging activities as assessed by ORAC assay were in the following order: blueberry juice > kale juice > mixed vegetable C juice > grape juice. Grape or blueberry juice showed strong abilities to prevent DNA damage in lymphocytes, and the difference between them was not significant according to the GSTM1/GSTT1 genotype. Conclusion: Antioxidant activities of fruit and vegetable juices and ex vivo DNA protective activity increased in the order of blueberry juice, grape juice, and kale juice, although the rankings were slightly different. Therefore, these juices rich in polyphenols and flavonoids deserve more attention for their high antioxidant capacity.
Go to : 
References
2. Rimm EB, Ascherio A, Giovannucci E, Spiegelman D, Stampfer MJ, Willett WC. Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men. JAMA. 1996; 275(6):447–451.

3. Boeing H, Bechthold A, Bub A, Ellinger S, Haller D, Kroke A, Leschik-Bonnet E, Müller MJ, Oberritter H, Schulze M, Stehle P, Watzl B. Critical review: vegetables and fruit in the prevention of chronic diseases. Eur J Nutr. 2012; 51(6):637–663.

5. Thomasset S, Berry DP, Cai H, West K, Marczylo TH, Marsden D, Brown K, Dennison A, Garcea G, Miller A, Hemingway D, Steward WP, Gescher AJ. Pilot study of oral anthocyanins for colorectal cancer chemoprevention. Cancer Prev Res (Phila). 2009; 2(7):625–633.

6. Lee HR, Jung BR, Park JY, Hwang IW, Kim SK, Choi JU, Lee SH, Chung SK. Antioxidant activity and total phenolic contents of grape juice products in the Korean market. Korean J Food Preserv. 2008; 15(3):445–449.
7. Jeong SM, Son MH, Lee SH. A survey on contents of phenolic compounds of market fruit and vegetables juices. J Basic Sci. 2003; 18:117–123.
8. Blumberg JB, Vita JA, Chen CY. Concord grape juice polyphenols and cardiovascular risk factors: dose-response relationships. Nutrients. 2015; 7(12):10032–10052.

9. Lichtenthäler R, Marx F. Total oxidant scavenging capacities of common European fruit and vegetable juices. J Agric Food Chem. 2005; 53(1):103–110.

10. Lee BH, Kim SY, Cho CH, Chung DK, Chun OK, Kim DO. Estimation of daily per capita intake of total phenolics, total flavonoids, and antioxidant capacities from fruit and vegetable juices in the Korean diet based on the Korea National Health and Nutrition Examination Survey 2008. Korean J Food Sci Technol. 2011; 43(4):475–482.

11. Chung HJ. Comparison of physicochemical properties and physiological activities of commercial fruit juices. Korean J Food Preserv. 2012; 19(5):712–719.

12. Lee MH, Kim MS, Shin HG, Sohn HY. Evaluation of antimicrobial, antioxidant, and antithrombin activity of domestic fruit and vegetable juice. Korean J Microbiol Biotechnol. 2011; 39(2):146–152. 152.
13. Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005; 53(6):1841–1856.

14. Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005; 53(10):4290–4302.

15. Chen HM, Muramoto K, Yamauchi F, Fujimoto K, Nokihara K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J Agric Food Chem. 1998; 46(1):49–53.

16. Ghiselli A, Serafini M, Maiani G, Azzini E, Ferro-Luzzi A. A fluorescence-based method for measuring total plasma antioxidant capability. Free Radic Biol Med. 1995; 18(1):29–36.

17. Aruoma OI, Halliwell B, Williamson G. In vitro methods for characterizing potential prooxidant and antioxidant actions of nonnutritive substances in plant foods. Aruoma OI, Cuppet SL, editors. editors.Antioxidant Methodology: In Vivo and In Vitro Concepts. Champaign (IL): AOCS Press;1997. p. 173–184.
18. Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988; 175(1):184–191.

19. Park EJ, Kang MH. Application of the alkaline comet assay for detecting oxidative DNA damage in human biomonitoring. Korean J Nutr. 2002; 35(2):213–222.
20. Strange RC, Spiteri MA, Ramachandran S, Fryer AA. Glutathione-S-transferase family of enzymes. Mutat Res. 2001; 482(1–2):21–26.

21. Bell DA, Taylor JA, Paulson DF, Robertson CN, Mohler JL, Lucier GW. Genetic risk and carcinogen exposure: a common inherited defect of the carcinogen-metabolism gene glutathione S-transferase M1 (GSTM1) that increases susceptibility to bladder cancer. J Natl Cancer Inst. 1993; 85(14):1159–1164.

22. Pemble S, Schroeder KR, Spencer SR, Meyer DJ, Hallier E, Bolt HM, Ketterer B, Taylor JB. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem J. 1994; 300(Pt 1):271–276.

23. Lemańska K, Szymusiak H, Tyrakowska B, Zieliński R, Soffers AE, Rietjens IM. The influence of pH on antioxidant properties and the mechanism of antioxidant action of hydroxyflavones. Free Radic Biol Med. 2001; 31(7):869–881.

24. Kim GD, Lee YS, Cho JY, Lee YH, Choi KJ, Lee Y, Han TH, Lee SH, Park KH, Moon JH. Comparison of the content of bioactive substances and the inhibitory effects against rat plasma oxidation of conventional and organic hot peppers (Capsicum annuum L.). J Agric Food Chem. 2010; 58(23):12300–12306.

25. Jeong CH, Choi SG, Heo HJ. Analysis of nutritional compositions and antioxidative activities of Korean commercial blueberry and raspberry. J Korean Soc Food Sci Nutr. 2008; 37(11):1375–1381.

26. Seeram NP, Aviram M, Zhang Y, Henning SM, Feng L, Dreher M, Heber D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J Agric Food Chem. 2008; 56(4):1415–1422.

27. Vanzani P, Rossetto M, De Marco V, Rigo A, Scarpa M. Efficiency and capacity of antioxidant rich foods in trapping peroxyl radicals: a full evaluation of radical scavenging activity. Food Res Int. 2011; 44(1):269–275.

28. Park YK, Kim JS, Kang MH. Concord grape juice supplementation reduces blood pressure in Korean hypertensive men: double-blind, placebo controlled intervention trial. Biofactors. 2004; 22(1–4):145–147.

29. Park YK, Lee SH, Park E, Kim JS, Kang MH. Changes in antioxidant status, blood pressure, and lymphocyte DNA damage from grape juice supplementation. Ann N Y Acad Sci. 2009; 1171(1):385–390.

30. Park YK, Park E, Kim JS, Kang MH. Daily grape juice consumption reduces oxidative DNA damage and plasma free radical levels in healthy Koreans. Mutat Res. 2003; 529(1–2):77–86.

31. Jeon EJ, Kim JS, Park YK, Kim TS, Kang MH. Protective effect of yellow-green vegetable juices on DNA damage in Chinese hamster lung cell using comet assay. Korean J Nutr. 2003; 36(1):24–31.
32. Kim HY, Park YK, Kim TS, Kang MH. The effect of green vegetable drink supplementation on cellular DNA damage and antioxidant status of Korean smokers. Korean J Nutr. 2006; 39(1):18–27.
33. Lee SM, Park KY, Rhee SH. Antimutagenic effect and active compound analysis of kale juice in salmonella assay system. J Korean Soc Food Sci Nutr. 1997; 26(5):965–971.
34. Chung SY, Kim HW, Yoon S. Analysis of antioxidant nutrients in green yellow vegetable juice. Korean J Food Sci Technol. 1999; 31(4):880–886.
35. Chung SY, Kim NK, Yoon S. Nitrite scavenging effect of methanol fraction obtained from green yellow vegetable juices. J Korean Soc Food Sci Nutr. 1999; 28(2):342–347.
Go to : 
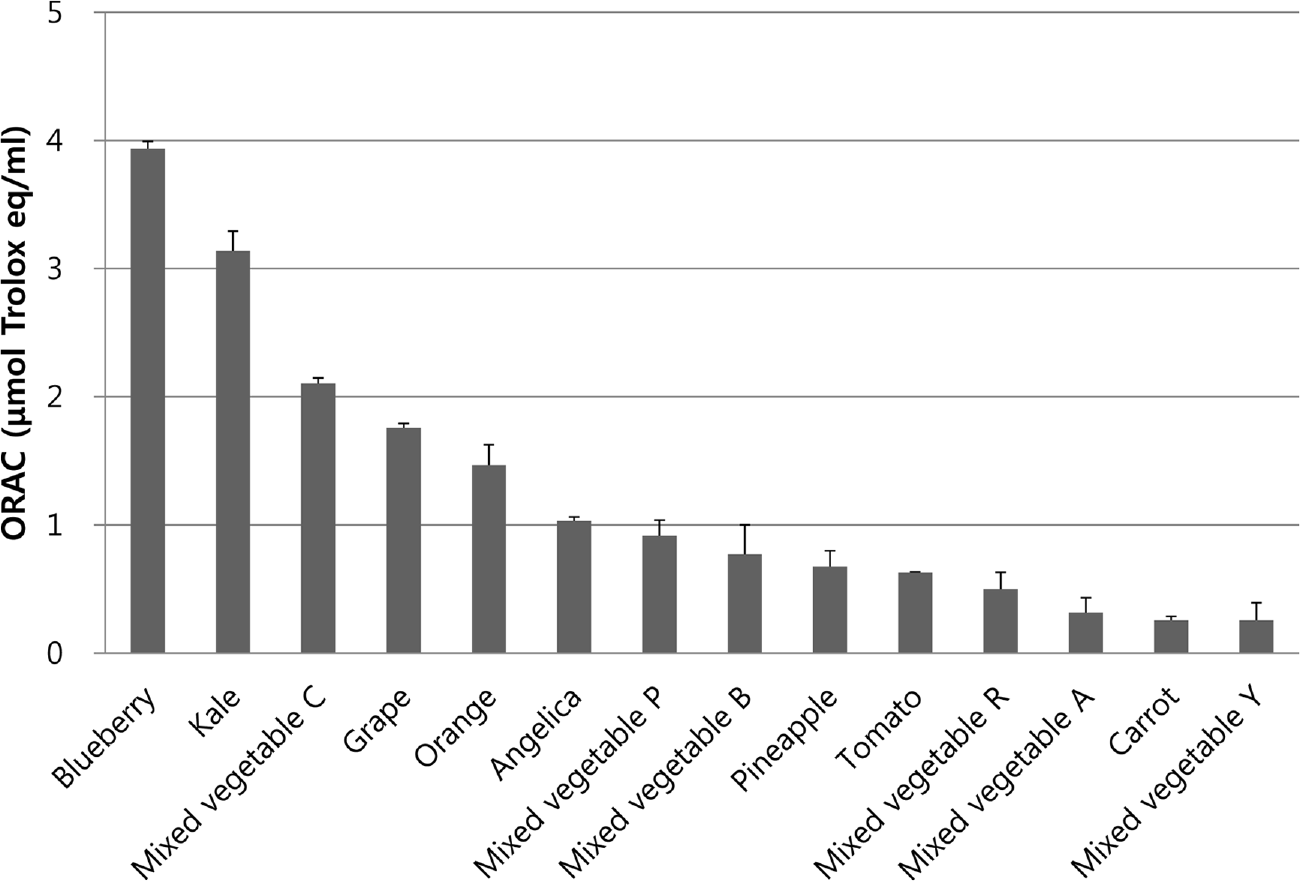 | Fig. 1.Comparison of ORAC values of ethanol extracts of commercial vegetable and fruit juices in Korea. Each bar represents the mean value with standard deviation. |
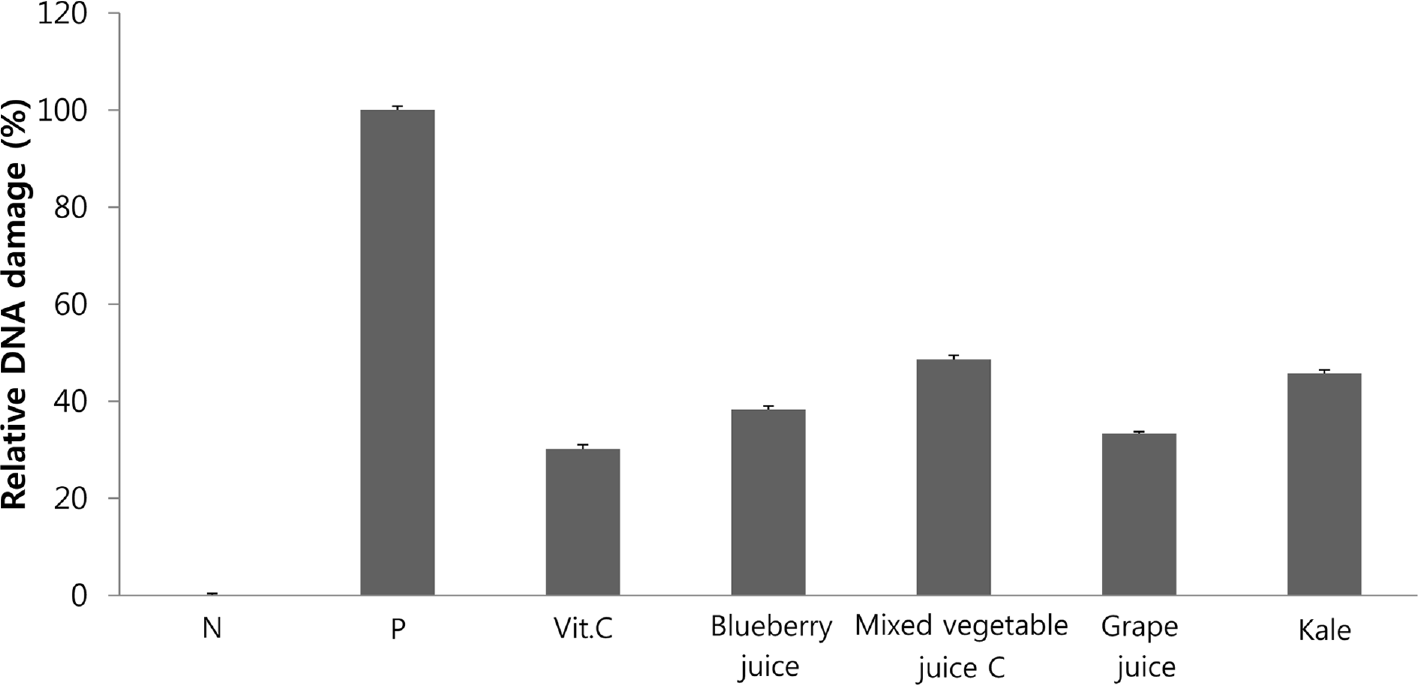 | Fig. 2.The ex vivo effects of vitamin C, blueberry juice, mixed vegetable C juice, kale juice, and grape juice on H2O2-induced DNA damage in human lymphocytes. Each bar represents the mean value with standard deviation. Each DNA damage (%) was calculated using tail moment by comparing with positive control. Vitamin C was used as a reference pure compound. N; negative control, P; positive control |
Table 2.
Antioxidant capacity of commercial fruit and vegetable juices in Korea1)
Table 3.
Levels of lymphocyte DNA damage expressed as TD, TL, TM of vitamin C, blueberry, mixed vegetable C, kale, and grape juice according to GSTM1 and T1 polymorphism
| GSTM1/ | DNA | N2) | P3) | Vitamin C |
Juices |
|||
|---|---|---|---|---|---|---|---|---|
| GSTT11) | damage | Blueberry | Mixed vegetable C | Kale | Grape | |||
| TD4) | 7.2 ± 0.2a7) | 16.3 ± 0.3d | 8.1 ± 0.2b | 8.9 ± 0.4bc | 9.5 ± 0.2c | 9.20 ± 0.3c | 8.7 ± 0.3bc | |
| +/+ | TL5) | 53.5 ± 2.3a | 165.4 ± 15.8d | 89.6 ± 3.2b | 101.1 ± 3.9bc | 114.2 ± 5.9c | 111.4 ± 6.4bc | 103.5 ± 5.8bc |
| TM6) | 4.3 ± 0.6a | 28.1 ± 0.3c | 8.8 ± 0.6b | 10.7 ± 0.6b | 11.8 ± 0.8b | 11.7 ± 0.8b | 10.0 ± 0.7b | |
| TD | 7.4 ± 0.3a | 16.6 ± 0.2e | 8.3 ± 0.6ab | 9.0 ± 0.4bc | 10.6 ± 0.4d | 9.6 ± 0.2cd | 8.9 ± 0.2bc | |
| +/- | TL | 58.34 ± 3.9a | 165.9 ± 5.7e | 83.7 ± 4.8b | 103.3 ± 4.2c | 121.2 ± 5.0d | 123.2 ± 6.0d | 97.8 ± 2.6c |
| TM | 4.8 ± 0.4a | 28.2 ± 0.8e | 8.5 ± 0.9b | 10.8 ± 0.7c | 13.7 ± 0.9d | 12.9 ± 0.7d | 9.4 ± 0.4bc | |
| TD | 7.0 ± 0.2a | 16.3 ± 0.3d | 8.1 ± 0.4b | 9.0 ± 0.4b | 10.2 ± 0.2c | 10.4 ± 0.4c | 8.9 ± 0.6b | |
| –/+ | TL | 49.8 ± 1.8a | 165.6 ± 9.7e | 91.8 ± 7.9b | 96.4 ± 9.3bc | 133.1 ± 7.2d | 1201 ± 5.5cd | 106.8 ± 11.4bc |
| TM | 3.9 ± 0.2a | 27.7 ± 1.5e | 8.6 ± 1.0b | 9.9 ± 0.9bc | 14.2 ± 0.9d | 13.1 ± 0.9cd | 11.1 ± 1.6bcd | |
| TD | 8.5 ± 0.6a | 16.8 ± 0.3c | 7.9 ± 0.3a | 9.4 ± 0.6a | 9.8 ± 0.5ab | 11.7 ± 1.5b | 8.8 ± 0.3a | |
| –/- | TL | 55.3 ± 4.1a | 164.2 ± 5.0e | 93.6 ± 4.8b | 100.2 ± 9.1b | 124.4 ± 7.2cd | 133.1 ± 10.1d | 107.9 ± 8.3bc |
| TM | 5.2 ± 0.6a | 28.4 ± 1.0e | 8.6 ± 0.3ab | 11.2 ± 1.4bc | 13.8 ± 1.4cd | 17.3 ± 3.8d | 10.4 ± 0.9bc | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download