Abstract
Purpose
This study was performed to compare total phenolic contents, in vitro antioxidant capacity, and reduction effect of Korean food groups on ex vivo DNA damage in human cells and analyze correlations between each indicator.
Methods
Vegetable foods in the Korean diet based the results of the KNHANES V-2 (2011) were classified into 10 food groups: cereals, fruits, vegetables, nuts, kimchi, seaweeds, potatoes, mushrooms, legumes, and oils. Eighty-four foods constituted more than 1% of the total intake in each food group and finally designated as vegetable foods in the Korean diet. Total phenolic content of each food group was measured. Further, in vitro antioxidant capacity was measured based on DPPH radical scavenging assay, TEAC assay, and ORACROO• assay. Ex vivo DNA damage in human lymphocytes was assessed using comet assay.
Results
Total phenolic contents of food groups of the Korean diet increased in the order of mushrooms, fruits, vegetables, seaweeds, and kimchi. Meanwhile, antioxidant rankings of food groups as mean values from the three in vitro test methods increased in the order of mushrooms, seaweeds, vegetables, kimchi, and fruits. Protection against ex vivo DNA damage in human lymphocytes was highest in mushrooms, followed by vegetables, fruits, seaweeds, and kimchi. The rankings of the food groups for total phenolic content, in vitro DAC, and ex vivo DNA protection activity were similar, and correlations between each indicator were significantly high.
Conclusion
Mushrooms, fruits, vegetables, and seaweeds among the tested food groups in the Korean diet showed high total phenolic contents, in vitro antioxidant capacities, and protection against DNA damage. Correlations between each indicator in terms of total phenolic content, in vitro antioxidant capacity, and ex vivo DNA protection between each food group were found to be particularly high.
Figures and Tables
 | Fig. 1Total phenolic content and antioxidant capacity of plant foods based on the dry mater of the edible part in the Korean diet. Different letters are significantly different among groups by Duncan’s multiple range test. Total: mixture of 10 Korean plant food groups |
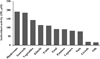 | Fig. 2Comparison of in vitro antioxidant activity of plant foods in Korean diet. Total: mixture of 10 Korean plant food groups |
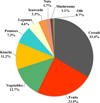 | Fig. 3Contribution of total antioxidant capacities (mean of DPPH, ORAC and TEAC) from all plant foods as a percentage of total dietary antioxidant capacities (TDAC) from per capita daily intake in the Korean diet (KNHANES V-2, 2011). The TDAC values were obtained by multiplying the total antioxidant capacity of each food group and the intake of each food. |
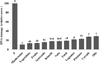 | Fig. 4Relative score of lymphocyte DNA damage by Comet assay expressed as tail moment (TM) of different food groups in Korean diet. Different letters are significantly different among groups by Duncan’s multiple range test (p < 0.05). P: positive control (H2O2) Total: mixture of 10 Korean plant food groups |
Table 2
Total dietary antioxidant capacity (TDAC) of food based on per capita daily intake in the Korean diet

Table 3
Protective effect on lymphocyte DNA damage by Comet assay expressed as TM, TL, TD of food groups in whole blood
References
1. De Bont R, van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis. 2004; 19(3):169–185.

3. Saura-Calixto F, Goñi I. Definition of the Mediterranean diet based on bioactive compounds. Crit Rev Food Sci Nutr. 2009; 49(2):145–152.

4. Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen elderly study. Lancet. 1993; 342(8878):1007–1011.

5. Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic S, Pekkarinen M, Simic BS, Toshima H, Feskens EJ, Hollman PC, Katan MB. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med. 1995; 155(4):381–386.
6. Doll R. An overview of the epidemiological evidence linking diet and cancer. Proc Nutr Soc. 1990; 49(2):119–131.

7. Hertog MG, van Poppel G, Verhoeven D. Potentially anticarcinogenic secondary metabolites from fruit and vegetables. In : Tomás-Barberán FA, Robins RJ, editors. Phytochemistry of Fruit and Vegetable. Oxford: Clarendon Press;1997. p. 313–329.
8. Serafini M, Del Rio D, Crozier A, Benzie IF. Effect of changes in fruit and vegetable intake on plasma antioxidant defenses in humans. Am J Clin Nutr. 2005; 81(2):531–532.

9. Mullen W, Marks SC, Crozier A. Evaluation of phenolic compounds in commercial fruit juices and fruit drinks. J Agric Food Chem. 2007; 55(8):3148–3157.

10. Luthria DL, Pastor-Corrales MA. Phenolic acids content of fifteen dry edible bean (Phaseolus vulgaris L.) varieties. J Food Compost Anal. 2006; 19(2-3):205–211.

11. Wang S, Meckling KA, Marcone MF, Kakuda Y, Tsao R. Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. J Agric Food Chem. 2011; 59(3):960–968.

12. Lila MA. Interactions between flavonoids that benefit human health. In : Gould K, Davies KM, Winefield C, editors. Anthocyanins: Biosynthesis, Functions, and Application. . New York (NY): Springer;2009. p. 305–320.
13. Yang M, Chung SJ, Chung CE, Kim DO, Song WO, Koo SI, Chun OK. Estimation of total antioxidant capacity from diet and supplements in US adults. Br J Nutr. 2011; 106(2):254–263.

14. Serafini M, Del Rio D. Understanding the association between dietary antioxidants, redox status and disease: is the total antioxidant capacity the right tool. Redox Rep. 2004; 9(3):145–152.

15. Hermsdorff HH, Puchau B, Volp AC, Barbosa KB, Bressan J, Zulet MÁ, Martínez JA. Dietary total antioxidant capacity is inversely related to central adiposity as well as to metabolic and oxidative stress markers in healthy young adults. Nutr Metab (Lond). 2011; 8:59.

16. Wang Y, Yang M, Lee SG, Davis CG, Koo SI, Chun OK. Dietary total antioxidant capacity is associated with diet and plasma antioxidant status in healthy young adults. J Acad Nutr Diet. 2012; 112(10):1626–1635.

17. Rockett JC, Burczynski ME, Fornace AJ Jr, Herrmann PC, Krawetz SA, Dix DJ. Surrogate tissue analysis: monitoring toxicant exposure and health status of inaccessible tissues through the analysis of accessible tissues and cells. Toxicol Appl Pharmacol. 2004; 194(2):189–199.

18. Collins A, Dusinská M, Franklin M, Somorovská M, Petrovská H, Duthie S, Fillion L, Panayiotidis M, Raslová K, Vaughan N. Comet assay in human biomonitoring studies: reliability, validation, and applications. Environ Mol Mutagen. 1997; 30(2):139–146.

19. Suh JH, Paek OJ, Kang YW, Ahn JE, Yun JS, Oh KS, An YS, Park SH, Lee SJ. Study on the antioxidant activity in the various vegetables. J Food Hyg Saf. 2013; 28(4):337–341.

20. Jeon EJ, Park YK, Kim JS, Kang MH. Comparison of the protective effect of antioxidant vitamins and fruits or vegetable juices on DNA damage in human lymphocyte cells using the comet assay. Korean J Nutr. 2004; 37(6):440–447.
21. Han JH, Lee HJ, Cho MR, Chang NS, Kim YR, Oh SY, Kang MH. Total antioxidant capacity of the Korean diet. Nutr Res Pract. 2014; 8(2):183–191.

22. Randhir R, Shetty P, Shetty K. L-DOPA and total phenolic stimulation in dark germinated fava bean in response to peptide and phytochemical elicitors. Process Biochem. 2002; 37(11):1247–1256.

23. Chen HM, Muramoto K, Yamauchi F, Fujimoto K, Nokihara K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J Agric Food Chem. 1998; 46(1):49–53.

24. Lee YJ, Lee SW, Lee SC, Park EJ. Antioxidant activities and antigenotoxic effect of ethanol extracts of Acorus gramineus, Bud of Aralica elata Seem, Capsella bursa-pastoris, and Taraxacum officinale. J Basic Sci. 2014; 31:45–58.
25. Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988; 175(1):184–191.

26. Park YK, Kim JS, Jeon EJ, Kang MH. The improvement of chaga mushroom (Inonotus obliquus) extract supplementation on the blood glucose and cellular DNA damage in streptozotocin-induced diabetic rats. Korean J Nutr. 2009; 42(1):5–13.
27. Saura-Calixto F, Goñi I. Antioxidant capacity of the Spanish Mediterranean diet. Food Chem. 2006; 94(3):442–447.

28. Choi SJ, Lee YS, Kim JK, Ki JK, Lim SS. Physiological activities of extract form edible mushrooms. J Korean Soc Food Sci Nutr. 2010; 39(8):1087–1096.
29. Qi Y, Zhao X, Lim YI, Park KY. Antioxidant and anticancer effects of edible and medicinal mushrooms. J Korean Soc Food Sci Nutr. 2013; 42:655–662.

30. Song YS, Kim SH, Sa JH, Jin C, Lim CJ, Park EH. Anti-angiogenic, antioxidant and xanthine oxidase inhibition activities of the mushroom Phellinus linteus. J Ethnopharmacol. 2003; 88(1):113–116.

31. Kim HY, Koo SC, Kang BK, Lee YH, Kim HT, Yun HT, Baek IY, Jeong HS, Choi MS. Growth characteristics of sprouts and changes of antioxidant activities in common bean (Phaseolus vulgaris L.) with cultivated temperature. Korean J Crop Sci. 2014; 59(2):201–207.

32. Woo KS, Seo HI, Lee YH, Kim HY, Ko JY, Song SB, Lee JS, Jung KY, Nam MH, Oh IS, Jeong HS. Antioxidant compounds and antioxidant activities of sweet potatoes with cultivated conditions. J Korean Soc Food Sci Nutr. 2012; 41(4):519–525.

33. Kim SM, Na MS. A study on skin care effects of rapeseed meal extract. KSBB J. 2013; 28(3):177–184.

34. Lee MH, Kim JM, Park EJ. Antioxidant and antigenotoxic effect of Sansuyu fruit (Corni fructus) extracted with various solvents. Cancer Prev Res. 2013; 18(1):66–73.
35. Jayakumar R, Kanthimathi MS. Dietary spices protect against hydrogen peroxide-induced DNA damage and inhibit nicotine-induced cancer cell migration. Food Chem. 2012; 134(3):1580–1584.

36. Madrigal-Bujaidar E, Diaz Barriga S, Mota P, Guzman R, Cassani M. Sister chromatid exchanges induced in vitro and in vivo by an extract of black pepper. Food Chem Toxicol. 1997; 35(6):567–571.

37. Kim SH, Kim MS, Lee MS, Park YS, Lee HJ, Kang SA, Lee HS, Lee KE, Yang HJ, Kim MJ, Lee YE, Kwon DY. Korean diet (K-diet): characteristics and historical background. J Ethn Food. 2016; 3(1):26–31.
38. Mellen PB, Walsh TF, Herrington DM. Whole grain intake and cardiovascular disease: a meta-analysis. Nutr Metab Cardiovasc Dis. 2008; 18(4):283–290.

39. Fung TT, Hu FB, Pereira MA, Liu S, Stampfer MJ, Colditz GA, Willett WC. Whole-grain intake and the risk of type 2 diabetes: a prospective study in men. Am J Clin Nutr. 2002; 76(3):535–540.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download