Abstract
Purpose
Ultraviolet (UV) irradiation decreases epidermal hydration, which is maintained by reduction of natural moisturizing factors (NMFs). Among various NMFs, free amino acids (AA) are major constituents generated by filaggrin degradation. This experiment was conducted to determine whether or not dietary supplementation of green tea extract (GTE) in UV-irradiated mice can improve epidermal levels of hydration, filaggrin, free AAs, and peptidylarginine deiminase-3 (PAD3) expression (an enzyme involved in filaggrin degradation).
Methods
Hairless mice were fed a diet of 1% GTE for 10 weeks in parallel with UV irradiation (group UV+1%GTE). As controls, hairless mice were fed a control diet in parallel with (group UV+) or without (group UV-) UV irradiation.
Results
In group UV+, epidermal levels of hydration and filaggrin were lower than those in group UV-; these levels increased in group UV+1% GTE to levels similar to group UV-. Epidermal levels of PAD3 and major AAs of NMF, alanine, glycine and serine were similar in groups UV- and UV+, whereas these levels highly increased in group UV+1% GTE.
Figures and Tables
 | Fig. 1Altered epidermal hydration of groups. Hairless mice fed a control diet without UV irradiation for 10 weeks (group UV-); UV-irradiated hairless mice fed a control diet (group UV+) or a diet supplemented with 1.0% green tea extract (group UV+1%GTE) for 10 weeks. Values are mean ± SD (n = 5). Values with different alphabetical letters are significantly different (p < 0.05) using one-way ANOVA and Tukey's multiple range test. |
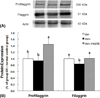 | Fig. 2Altered protein levels of profilaggrin and filaggrin in the epidermis of groups. Hairless mice fed a control diet without UV irradiation for 10 weeks (group UV-): UV-irradiated hairless mice fed a control diet (group UV+) or a diet supplemented with 1.0% green tea extract (group UV+1%GTE) for 10 weeks (A) Representative expression of profilaggrin and filaggrin proteins in the epidermis of groups (B) Signal intensities from multiple experiments of (A) were quantified and the integrated areas were normalized, first to the corresponding value of actin and then to the signal observed in the normal control group (group UV-). Values are mean ± SD (n = 5). Values with different alphabetical letters in profilaggrin and filaggrin are significantly different (p < 0.05) using one-way ANOVA and Tukey's multiple range test. |
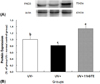 | Fig. 3Altered protein level of peptidylarginine deiminase-3 in the epidermis of groups. Hairless mice fed a control diet without UV irradiation for 10 weeks (group UV-); UV-irradiated hairless mice fed a control diet (group UV+) or a diet supplemented with 1.0% green tea extract (group UV+1%GTE) for 10 weeks (A) Representative expression of peptidylarginine deiminase-3 (PAD3) protein in the epidermis of groups (B) Signal intensities from multiple experiments of (A) were quantified and the integrated areas were normalized, first to the corresponding value of actin and then to the signal observed in the normal control group (group UV-). Values are mean ± SD (n = 5). Values with different alphabetical letters in PAD3 is significantly different (p < 0.05) using one-way ANOVA and Tukey's multiple range test. |
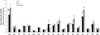 | Fig. 4Free amino acid contents in the epidermis of groups. Hairless mice fed a control diet without UV irradiation for 10 weeks (group UV-); UV-irradiated hairless mice fed a control diet (group UV+) or a diet supplemented with 1.0% green tea extract (group UV+1%GTE) for 10 weeks. Data are mean ± SD (n = 5). Values with different alphabetical letters in each amino acids are significantly different (p < 0.05) using one-way ANOVA and Tukey’s multiple range test. |
Table 1
Diet composition and UV irradiation of experimental groups (g/kg)

1) Groups UV- and UV+, hairless mice fed a control diet without (group UV-) or with (group UV+) UV irradiation for 10 weeks; Group GTE, hairless mice fed a diet containing 1.0% green tea extract (GTE) in parallel with UV irradiation for 10 weeks. 2) GTE: green tea extract 3) AIN-93 vitamin mix #310025 (Dyets Inc., Bethlehem, PA) 4) AIN-93G salt mix #210025 (Dyets Inc., Bethlehem, PA) 5) Casein (nitrogen × 6.25), 870 g/kg
References
1. Rawlings AV, Harding CR. Moisturization and skin barrier function. Dermatol Ther. 2004; 17:Suppl 1. 43–48.

2. Baroni A, Buommino E, De Gregorio V, Ruocco E, Ruocco V, Wolf R. Structure and function of the epidermis related to barrier properties. Clin Dermatol. 2012; 30(3):257–262.

3. Verdier-Sévrain S, Bont F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermatol. 2007; 6(2):75–82.

4. Sandilands A, Sutherland C, Irvine AD, McLean WH. Filaggrin in the frontline: role in skin barrier function and disease. J Cell Sci. 2009; 122(Pt 9):1285–1294.

5. Nachat R, Méchin MC, Takahara H, Chavanas S, Charveron M, Serre G, Simon M. Peptidylarginine deiminase isoforms 1-3 are expressed in the epidermis and involved in the deimination of K1 and filaggrin. J Invest Dermatol. 2005; 124(2):384–393.

6. Gilchrest BA. Skin aging and photoaging: an overview. J Am Acad Dermatol. 1989; 21(3 Pt 2):610–613.

8. Tagami H. Functional characteristics of the stratum corneum in photoaged skin in comparison with those found in intrinsic aging. Arch Dermatol Res. 2008; 300:Suppl 1. S1–S6.

9. Gruber JV, Holtz R. Examining communication between ultraviolet (UV)-damaged cutaneous nerve cells and epidermal keratinocytes in vitro. Toxicol Ind Health. 2009; 25(4-5):225–230.

10. Hwang E, Sun ZW, Lee TH, Shin HS, Park SY, Lee DG, Cho BG, Sohn H, Kwon OW, Kim SY, Yi TH. Enzyme-processed Korean Red Ginseng extracts protects against skin damage induced by UVB irradiation in hairless mice. J Ginseng Res. 2013; 37(4):425–434.

11. Kim J, Lee Y, Cho Y. Effects of dietary royal jelly on epidermal generation of ceramides from acidic sphingomyelin and glucosylceramides in UV-irradiated hairless mice. Curr Top Nutraceutical Res. 2012; 10(3/4):151–164.
12. Khan SG, Katiyar SK, Agarwal R, Mukhtar H. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: possible role in cancer chemoprevention. Cancer Res. 1992; 52(14):4050–4052.
13. Min J, Lee Y, Han SM, Choi Y. Dietary effect of royal jelly supplementation on epidermal levels of hydration, filaggrins, free amino acids and the related enzyme expression in UV irradiated hairless mice. Korean J Nutr. 2013; 46(2):109–118.

14. Katiyar SK, Elmets CA. Green tea polyphenolic antioxidants and skin photoprotection (Review). Int J Oncol. 2001; 18(6):1307–1313.

15. Jackson JK, Zhao J, Wong W, Burt HM. The inhibition of collagenase induced degradation of collagen by the galloyl-containing polyphenols tannic acid, epigallocatechin gallate and epicatechin gallate. J Mater Sci Mater Med. 2010; 21(5):1435–1443.

16. Hsu S, Bollag WB, Lewis J, Huang Q, Singh B, Sharawy M, Yamamoto T, Schuster G. Green tea polyphenols induce differentiation and proliferation in epidermal keratinocytes. J Pharmacol Exp Ther. 2003; 306(1):29–34.

17. Kiss I, Chen S, Tramposch KM. The effect of high and low ultraviolet-B dose exposure on the degree of hairless mouse skin wrinkling. Photochem Photobiol. 1991; 53(1):109–112.

18. Takami S, Imai T, Hasumura M, Cho YM, Onose J, Hirose M. Evaluation of toxicity of green tea catechins with 90-day dietary administration to F344 rats. Food Chem Toxicol. 2008; 46(6):2224–2229.

19. Lee B, Kim J, Hwang J, Cho Y. Dietary effect of green tea extract on epidermal levels of skin pH related factors, lactate dehydrogenase protein expression and activity in UV-irradiated hairless mice. J Nutr Health. 2016; 49(2):63–71.

20. Ginger RS, Blachford S, Rowland J, Rowson M, Harding CR. Filaggrin repeat number polymorphism is associated with a dry skin phenotype. Arch Dermatol Res. 2005; 297(6):235–241.

21. Dale BA, Resing KA, Lonsdale-Eccles JD. Filaggrin: a keratin filament associated protein. Ann N Y Acad Sci. 1985; 455:330–342.

22. Kanno T, Kawada A, Yamanouchi J, Yosida-Noro C, Yoshiki A, Shiraiwa M, Kusakabe M, Manabe M, Tezuka T, Takahara H. Human peptidylarginine deiminase type III: molecular cloning and nucleotide sequence of the cDNA, properties of the recombinant enzyme, and immunohistochemical localization in human skin. J Invest Dermatol. 2000; 115(5):813–823.

23. Steinert PM, Cantieri JS, Teller DC, Lonsdale-Eccles JD, Dale BA. Characterization of a class of cationic proteins that specifically interact with intermediate filaments. Proc Natl Acad Sci U S A. 1981; 78(7):4097–4101.

24. Visscher M, Robinson M, Wickett R. Stratum corneum free amino acids following barrier perturbation and repair. Int J Cosmet Sci. 2011; 33(1):80–89.

25. Yamada Y, Obayashi M, Ishikawa T, Kiso Y, Ono Y, Yamashita K. Dietary tocotrienol reduces UVB-induced skin damage and sesamin enhances tocotrienol effects in hairless mice. J Nutr Sci Vitaminol (Tokyo). 2008; 54(2):117–123.

26. Scott IR, Harding CR, Barrett JG. Histidine-rich protein of the keratohyalin granules. Source of the free amino acids, urocanic acid and pyrrolidone carboxylic acid in the stratum corneum. Biochim Biophys Acta. 1982; 719(1):110–117.
27. Kam E, Resing KA, Lim SK, Dale BA. Identification of rat epidermal profilaggrin phosphatase as a member of the protein phosphatase 2A family. J Cell Sci. 1993; 106(Pt 1):219–226.

28. Pearton DJ, Nirunsuksiri W, Rehemtulla A, Lewis SP, Presland RB, Dale BA. Proprotein convertase expression and localization in epidermis: evidence for multiple roles and substrates. Exp Dermatol. 2001; 10(3):193–203.

29. Jiang SJ, Chu AW, Lu ZF, Pan MH, Che DF, Zhou XJ. Ultraviolet B-induced alterations of the skin barrier and epidermal calcium gradient. Exp Dermatol. 2007; 16(12):985–992.

30. Jang SI, Steinert PM, Markova NG. Activator protein 1 activity is involved in the regulation of the cell type-specific expression from the proximal promoter of the human profilaggrin gene. J Biol Chem. 1996; 271(39):24105–24114.

31. Balasubramanian S, Efimova T, Eckert RL. Green tea polyphenol stimulates a Ras, MEKK1, MEK3, and p38 cascade to increase activator protein 1 factor-dependent involucrin gene expression in normal human keratinocytes. J Biol Chem. 2002; 277(3):1828–1836.

32. Hoste E, Kemperman P, Devos M, Denecker G, Kezic S, Yau N, Gilbert B, Lippens S, De Groote P, Roelandt R, Van Damme P, Gevaert K, Presland RB, Takahara H, Puppels G, Caspers P, Vandenabeele P, Declercq W. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011; 131(11):2233–2241.

33. Kim Y, Han SM, Cho Y. The dietary effect of royal jelly supplementation on epidermal levels of filaggrin and free amino acids during menopause in rats. J Korean Soc Food Sci Nutr. 2013; 42(3):389–396.

34. Hsu S, Yamamoto T, Borke J, Walsh DS, Singh B, Rao S, Takaaki K, Nah-Do N, Lapp C, Lapp D, Foster E, Bollag WB, Lewis J, Wataha J, Osaki T, Schuster G. Green tea polyphenol-induced epidermal keratinocyte differentiation is associated with coordinated expression of p57/KIP2 and caspase 14. J Pharmacol Exp Ther. 2005; 312(3):884–890.

35. Koyama J, Horii I, Kawasaki K, Nakayama Y, Morikawa Y, Mitsui T, Kumagai H. Free amino acids of stratum corneum as a biochemical marker to evaluate dry skin. J Soc Cosmet Chem. 1984; 35(4):183–195.
36. Tabachnick J, LaBadie JH. Studies on the biochemistry of epidermis. IV. The free amino acids, ammonia, urea, and pyrrolidone carboxylic acid content of conventional and germ-free albino guina pig epidermia. J Invest Dermatol. 1970; 54(1):24–31.
37. Joo KM, Han JY, Son ED, Nam GW, Jeong HJ, Lim KM, Cho JC. Study on the relationship between skin dryness and amino acids in stratum corneum. J Soc Cosmet Sci Korea. 2012; 38(1):75–82.

38. Bak H, Hong SP, Jeong SK, Choi EH, Lee SE, Lee SH, Ahn SK. Altered epidermal lipid layers induced by long-term exposure to suberythemal-dose ultraviolet. Int J Dermatol. 2011; 50(7):832–837.

39. Kim H, Oh I, Park KH, Kim NM, Do JH, Cho Y. Stimulatory effect of dietary red ginseng on epidermal hydration and ceramide levels in ultraviolet-irradiated hairless mice. J Med Food. 2009; 12(4):746–754.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download