Abstract
Purpose:
Genetic polymorphisms in antioxidant defense and detoxification genes may modulate the levels of oxidative stress biomarkers.
Methods:
A total of 301 healthy preschool-aged children in the Seoul and Kyunggi areas were recruited. DNA was extracted from blood for genotyping of manganese superoxide dismutase (Mn-SOD) Val16Ala, glutathione S-transferase (GST) P1 Ile105Val, GSTT1 present/null, and GSTM1 present/null polymorphisms by PCR-restriction fragment length polymorphism or multiplex PCR analyses. In addition to a questionnaire survey, the levels of urinary 8-hydroxyl-2-deoxiguanosine (8-OHdG) and plasma malondialdehyde (MDA) were measured by ELISA.
Results:
Significantly higher urinary 8-OHdG concentrations were observed in GSTP1 Ile/Val + Val/Val genotype (p = 0.030), and tended to be higher in Mn-SOD Val/Val genotype (p = 0.065). On the other hand, exposure to environmental tobacco smoking (ETS) and interaction between ETS and gene polymorphisms did not significantly influence either urinary 8-OHdG concentrations or serum MDA.
Go to : 
REFERENCES
1.Ames BN. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983. 221(4617):1256–1264.

2.Zhang H., Davies KJ., Forman HJ. Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med. 2015. 8(Pt B):314–336.

3.Thanan R., Oikawa S., Hiraku Y., Ohnishi S., Ma N., Pinlaor S., Yongvanit P., Kawanishi S., Murata M. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int J Mol Sci. 2015. 16(1):193–217.

4.Dikalov SI., Ungvari Z. Role of mitochondrial oxidative stress in hypertension. Am J Physiol Heart Circ Physiol. 2013. 305(10):H1417–H1427.

5.Yan MH., Wang X., Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med. 2013. 62:90–101.

6.Zhu H., Li YR. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: updated experimental and clinical evidence. Exp Biol Med (Maywood). 2012. 237(5):474–480.

7.Kim YJ., Kim EH., Hahm KB. Oxidative stress in inflammation-based gastrointestinal tract diseases: challenges and opportunities. J Gastroenterol Hepatol. 2012. 27(6):1004–1010.

8.Shimoda-Matsubayashi S., Matsumine H., Kobayashi T., Nakagawa-Hattori Y., Shimizu Y., Mizuno Y. Structural dimorphism in the mitochondrial targeting sequence in the human manganese superoxide dismutase gene. A predictive evidence for conformational change to influence mitochondrial transport and a study of allelic association in Parkinson's disease. Biochem Biophys Res Commun. 1996. 226(2):561–565.

9.Klaunig JE., Kamendulis LM., Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 2010. 38(1):96–109.

10.Bresciani G., Cruz IB., de Paz JA., Cuevas MJ., González-Gallego J. The MnSOD Ala16Val SNP: relevance to human diseases and interaction with environmental factors. Free Radic Res. 2013. 47(10):781–792.

11.Duarte MM., Moresco RN., Duarte T., Santi A., Bagatini MD., Da Cruz IB., Schetinger MR., Loro VL. Oxidative stress in hypercholesterolemia and its association with Ala16Val superoxide dismutase gene polymorphism. Clin Biochem. 2010. 43(13-14):1118–1123.

12.Knapen MF., Zusterzeel PL., Peters WH., Steegers EA. Glutathione and glutathione-related enzymes in reproduction. A review. Eur J Obstet Gynecol Reprod Biol. 1999. 82(2):171–184.
13.Çelįk SK., Aras N., Yildirim Ö., Turan F., Görür A., Yildirim H., Tamer L. Glutathione S-transferase GSTM 1, null genotype may be associated with susceptibility to age-related cataract. Adv Clin Exp Med. 2015. 24(1):113–119.
14.Goodrich JM., Basu N. Variants of glutathione s-transferase pi 1 exhibit differential enzymatic activity and inhibition by heavy metals. Toxicol In Vitro. 2012. 26(4):630–635.

15.Suvakov S., Damjanovic T., Stefanovic A., Pekmezovic T., Savic-Radojevic A., Pljesa-Ercegovac M., Matic M., Djukic T., Coric V., Jakovljevic J., Ivanisevic J., Pljesa S., Jelic-Ivanovic Z., Mimic-Oka J., Dimkovic N., Simic T. Glutathione S-transferase A1, M1, P1 and T1 null or low-activity genotypes are associated with enhanced oxidative damage among haemodialysis patients. Nephrol Dial Transplant. 2013. 28(1):202–212.

16.Brunst KJ., Baccarelli AA., Wright RJ. Integrating mitochondriom-ics in children's environmental health. J Appl Toxicol. 2015. 35(9):976–991.

17.Ahn K. The role of air pollutants in atopic dermatitis. J Allergy Clin Immunol. 2014. 134(5):993–999.

18.McCrindle BW. Cardiovascular consequences of childhood obesity. Can J Cardiol. 2015. 31(2):124–130.

19.Akyol O., Canatan H., Yilmaz HR., Yuce H., Ozyurt H., Sogut S., Gulec M., Elyas H. PCR/RFLP-based cost-effective identification of SOD2 signal (leader) sequence polymorphism (Ala-9Val) using NgoM IV: a detailed methodological approach. Clin Chim Acta. 2004. 345(1-2):151–159.

20.Wilson MH., Grant PJ., Hardie LJ., Wild CP. Glutathione S-transferase M1 null genotype is associated with a decreased risk of myocardial infarction. FASEB J. 2000. 14(5):791–796.

21.Sutton A., Khoury H., Prip-Buus C., Cepanec C., Pessayre D., Degoul F. The Ala16Val genetic dimorphism modulates the import of human manganese superoxide dismutase into rat liver mitochondria. Pharmacogenetics. 2003. 13(3):145–157.
22.Becer E., Çırakoğlu A. Association of the Ala16Val MnSOD gene polymorphism with plasma leptin levels and oxidative stress biomarkers in obese patients. Gene. 2015. 568(1):35–39.

23.Zimniak P., Nanduri B., Pikuła S., Bandorowicz-Pikuła J., Singhal SS., Srivastava SK., Awasthi S., Awasthi YC. Naturally occurring human glutathione S-transferase GSTP1-1 isoforms with isoleucine and valine in position 104 differ in enzymic properties. Eur J Biochem. 1994. 224(3):893–899.

24.Watson MA., Stewart RK., Smith GB., Massey TE., Bell DA. Human glutathione S-transferase P1 polymorphisms: relationship to lung tissue enzyme activity and population frequency distribution. Carcinogenesis. 1998. 19(2):275–280.

25.Aynacioglu AS., Nacak M., Filiz A., Ekinci E., Roots I. Protective role of glutathione S-transferase P1 (GSTP1) Val105Val genotype in patients with bronchial asthma. Br J Clin Pharmacol. 2004. 57(2):213–217.

26.Tamer L., Calikoğlu M., Ates NA., Yildirim H., Ercan B., Saritas E., Unlü A., Atik U. Glutathione-S-transferase gene polymorphisms (GSTT1, GSTM1, GSTP1) as increased risk factors for asthma. Respirology. 2004. 9(4):493–498.

27.Lee E., Chang HY., Lee KS., Suh DI., Yu HS., Kang MJ., Choi IA., Park J., Kim KW., Shin YH., Ahn KM., Kwon JY., Choi SJ., Lee KJ., Won HS., Yang SI., Jung YH., Kim HY., Seo JH., Kwon JW., Kim BJ., Kim HB., Lee SY., Kim EJ., Lee JS., Keyes KM., Shin YJ., Hong SJ. COCOA study group. The effect of perinatal anxiety on bronchiolitis is influenced by polymorphisms in ROS-related genes. BMC Pulm Med. 2014. 14:154.

28.Reddy P., Naidoo RN., Robins TG., Mentz G., London SJ., Li H., Naidoo R. GSTM1, GSTP1, and NQO1 polymorphisms and susceptibility to atopy and airway hyperresponsiveness among South African schoolchildren. Lung. 2010. 188(5):409–414.

29.Karam RA., Pasha HF., El-Shal AS., Rahman HM., Gad DM. Impact of glutathione-S-transferase gene polymorphisms on enzyme activity, lung function and bronchial asthma susceptibility in Egyptian children. Gene. 2012. 497(2):314–319.

30.Jo HR., Lee HJ., Kang MH. Antioxidative status, DNA damage and lipid profiles in Korean young adults by glutathione S-transferase polymorphisms. Korean J Nutr. 2011. 44(1):16–28.

31.Gergen PJ., Fowler JA., Maurer KR., Davis WW., Overpeck MD. The burden of environmental tobacco smoke exposure on the respiratory health of children 2 months through 5 years of age in the United States: Third National Health and Nutrition Examination Survey, 1988 to 1994. Pediatrics. 1998. 101(2):E8.

32.Yi O., Kwon HJ., Kim H., Ha M., Hong SJ., Hong YC., Leem JH., Sakong J., Lee CG., Kim SY., Kang D. Effect of environmental tobacco smoke on atopic dermatitis among children in Korea. Environ Res. 2012. 113:40–45.

33.Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention. Korean adults male smoking rate [Internet]. Cheongju: Ministry of Health and Welfare;2015. [cited 2015 Sep 8]. Available from:. http://kosis.kr.
34.Holloway JW., Savarimuthu Francis S., Fong KM., Yang IA. Genomics and the respiratory effects of air pollution exposure. Respirology. 2012. 17(4):590–600.

35.Wu J., Hankinson J., Kopec-Harding K., Custovic A., Simpson A. Interaction between glutathione S-transferase variants, maternal smoking and childhood wheezing changes with age. Pediatr Allergy Immunol. 2013. 24(5):501–508.
36.Chiou CC., Chang PY., Chan EC., Wu TL., Tsao KC., Wu JT. Urinary 8-hydroxydeoxyguanosine and its analogs as DNA marker of oxidative stress: development of an ELISA and measurement in both bladder and prostate cancers. Clin Chim Acta. 2003. 334(1-2):87–94.

37.Forlenza MJ., Miller GE. Increased serum levels of 8-hydroxy-2'-deoxyguanosine in clinical depression. Psychosom Med. 2006. 68(1):1–7.

38.Carvalho AM., Miranda AM., Santos FA., Loureiro AP., Fisberg RM., Marchioni DM. High intake of heterocyclic amines from meat is associated with oxidative stress. Br J Nutr. 2015. 113(8):1301–1307.

39.Il'yasova D., Scarbrough P., Spasojevic I. Urinary biomarkers of oxidative status. Clin Chim Acta. 2012. 413(19-20):1446–1453.
40.Soto-Méndez MJ., Aguilera CM., Campaña-Martín L., Martín-Laguna V., Schümann K., Solomons NW., Gil A. Variation in hydration status within the normative range is associated with urinary biomarkers of systemic oxidative stress in Guatemalan preschool children. Am J Clin Nutr. 2015. 102(4):865–872.

41.Oztop D., Altun H., Baskol G., Ozsoy S. Oxidative stress in children with attention deficit hyperactivity disorder. Clin Biochem. 2012. 45(10-11):745–748.

42.Al-Alem U., Gann PH., Dahl J., van Breemen RB., Mistry V., Lam PM., Evans MD., Van Horn L., Wright ME. Associations between functional polymorphisms in antioxidant defense genes and urinary oxidative stress biomarkers in healthy, premenopausal women. Genes Nutr. 2012. 7(2):191–195.

43.Prasad SB., Vidyullatha P., Vani GT., Devi RP., Rani UP., Reddy PP., Prasad HM. Association of gene polymorphism in detoxification enzymes and urinary 8-OHdG levels in traffic policemen exposed to vehicular exhaust. Inhal Toxicol. 2013. 25(1):1–8.
Go to : 
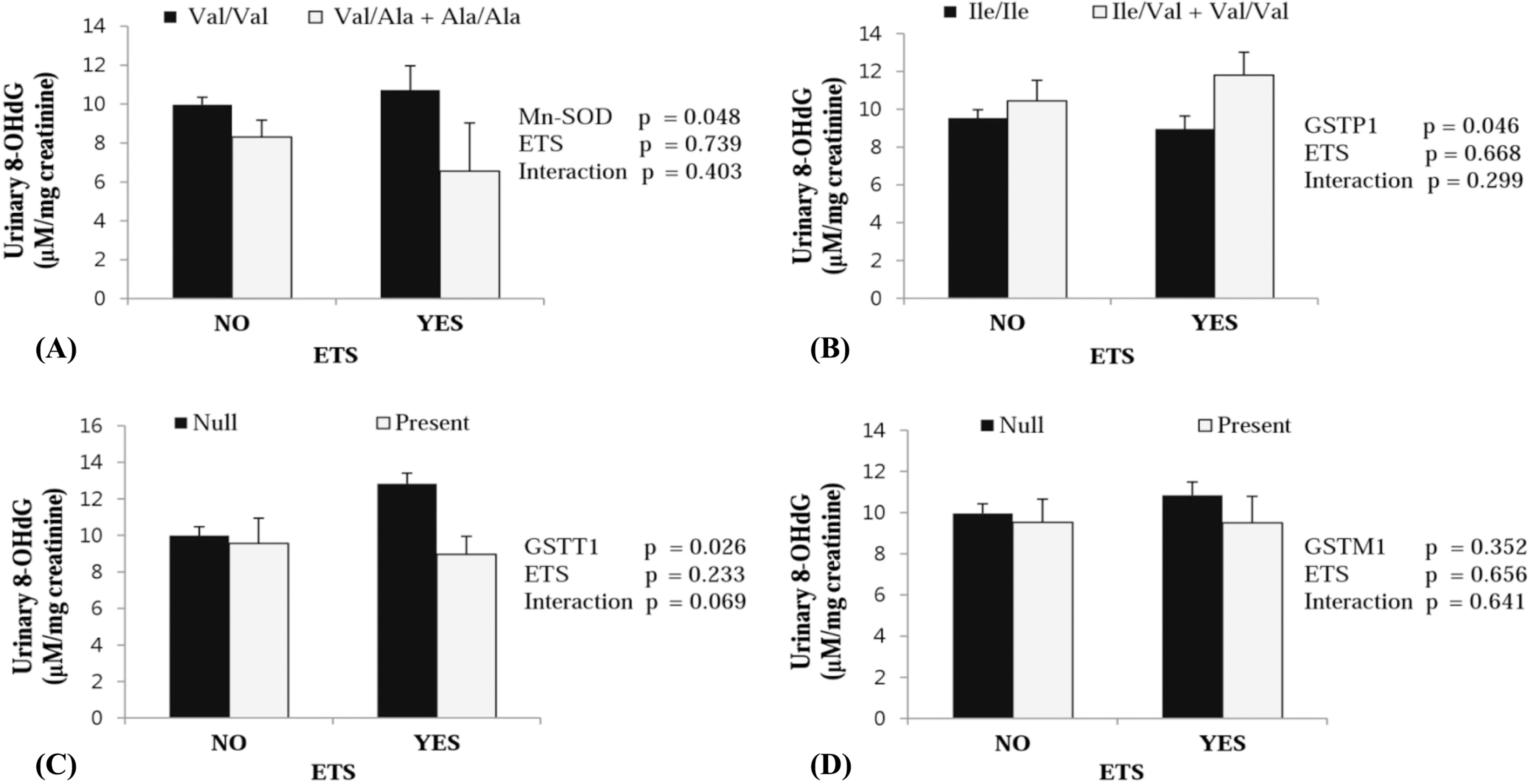 | Fig. 1.The levels of urinary 8-hydroxyl-2-deoxyguanosine (8-OHdG) according to exposure to environmental tobacco smoking (ETS) and gene polymorphisms. [(A) Mn-SOD, (B) GSTP1, (C) GSTT1, and (D) GSTM1] Data were analyzed using GLM models adjusted for age, sex, and nutrient supplement intake. |
Table 1.
General characteristics of the study population
Table 2.
Genotype distributions and allelic frequencies for Mn SOD, GSTP1, GSTT1, and GSTM1 gene polymorphisms in the stud population
Table 3.
Concentrations of oxidative stress markers according to polymorphisms




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download