Abstract
Purpose:
We developed a method to load lycopene into maltodextrin and cyclodextrin in an attempt to overcome the poor bioavailability and improve the anti-inflammatory effect of this polyphenol.
Methods:
Nanosized lycopenes were encapsulated into biodegradable amphiphillic cyclodextrin and maltodextrin molecules prepared using a high pressure homogenizer at 15,000~25,000 psi. Cell damage was induced by lipopolysaccharides (LPS) in a mouse macrophage cell line, RAW 264.7. The cells were subjected to various doses of free lycopene (FL) and nanoencapsulated lycopene (NEL). RT-PCR was used to quantify the tumor necrosis factor (TNF-α), interleukin-1β (IL-1β), IL-6, inducible nitric oxide synthase (iNOS), and cyclooxigenase-2 (COX-2) mRNA levels, while ELISA was used to determine the protein levels of TNF-α, IL-1β, and IL-6.
Results:
NEL significantly reduced the mRNA expression of IL-6 and IL-1β at the highest dose, while not in cells treated with FL. In addition, NEL treatment caused a significant reduction in IL-6 and TNF-α protein levels, compared to cells treated with a similar dose of FL. In addition, mRNA expression of iNOS and COX-2 enzyme in the activated macrophages was more efficiently suppressed by NEL than by FL.
REFERENCES
1.Agarwal S., Rao AV. Tomato lycopene and its role in human health and chronic diseases. CMAJ. 2000. 163(6):739–744.
2.Giovannucci E., Rimm EB., Liu Y., Stampfer MJ., Willett WC. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002. 94(5):391–398.

3.Stahl W., Sies H. Lycopene: a biologically important carotenoid for humans? Arch Biochem Biophys. 1996. 336(1):1–9.

4.Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst. 1999. 91(4):317–331.

5.Giovannucci E., Ascherio A., Rimm EB., Stampfer MJ., Colditz GA., Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. 1995. 87(23):1767–1776.
6.Schuurman AG., Goldbohm RA., Dorant E., van den Brandt PA. Vegetable and fruit consumption and prostate cancer risk: a cohort study in The Netherlands. Cancer Epidemiol Biomarkers Prev. 1998. 7(8):673–680.
7.Chen L., Stacewicz-Sapuntzakis M., Duncan C., Sharifi R., Ghosh L., van Breemen R., Ashton D., Bowen PE. Oxidative DNA damage in prostate cancer patients consuming tomato sauce-based entrees as a whole-food intervention. J Natl Cancer Inst. 2001. 93(24):1872–1879.

8.Kucuk O., Sarkar FH., Sakr W., Djuric Z., Pollak MN., Khachik F., Li YW., Banerjee M., Grignon D., Bertram JS., Crissman JD., Pontes EJ., Wood DP Jr. Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol Biomarkers Prev. 2001. 10(8):861–868.
9.Michael McClain R., Bausch J. Summary of safety studies conducted with synthetic lycopene. Regul Toxicol Pharmacol. 2003. 37(2):274–285.

10.Borel P., Grolier P., Armand M., Partier A., Lafont H., Lairon D., Azais-Braesco V. Carotenoids in biological emulsions: solubility, surface-to-core distribution, and release from lipid droplets. J Lipid Res. 1996. 37(2):250–261.

11.Castenmiller JJ., West CE. Bioavailability and bioconversion of carotenoids. Annu Rev Nutr. 1998. 18(1):19–38.

12.Schierle J., Bretzel W., Bühler I., Faccin N., Hess D., Steiner K., Schüep W. Content and isomeric ratio of lycopene in food and human blood plasma. Food Chem. 1997. 59(3):459–465.

13.Shi J., Le Maguer M. Lycopene in tomatoes: chemical and physical properties affected by food processing. Crit Rev Food Sci Nutr. 2000. 40(1):1–42.

14.Britton G. Structure and properties of carotenoids in relation to function. FASEB J. 1995. 9(15):1551–1558.

15.Lee MT., Chen BH. Stability of lycopene during heating and illumination in a model system. Food Chem. 2002. 78(4):425–432.

16.Offord EA., Gautier JC., Avanti O., Scaletta C., Runge F., Krämer K., Applegate LA. Photoprotective potential of lycopene, beta-carotene, vitamin E, vitamin C and carnosic acid in UVA-irradiated human skin fibroblasts. Free Radic Biol Med. 2002. 32(12):1293–1303.
17.Mokarram R., Mortazavi S., Najafi MH., Shahidi F. The influence of multi stage alginate coating on survivability of potential probiotic bacteria in simulated gastric and intestinal juice. Food Res Int. 2009. 42(8):1040–1045.

18.Yu H., Huang Q. Enhanced in vitro anti-cancer activity of curcumin encapsulated in hydrophobically modified starch. Food Chem. 2010. 119(2):669–674.

19.Sahu A., Bora U., Kasoju N., Goswami P. Synthesis of novel biodegradable and self-assembling methoxy poly(ethylene glycol)-palmitate nanocarrier for curcumin delivery to cancer cells. Acta Biomater. 2008. 4(6):1752–1761.

20.Ma Z., Haddadi A., Molavi O., Lavasanifar A., Lai R., Samuel J. Micelles of poly(ethylene oxide)-b-poly(epsilon-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J Biomed Mater Res A. 2008. 86(2):300–310.
21.Wang X., Jiang Y., Wang YW., Huang MT., Ho CT., Huang Q. Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chem. 2008. 108(2):419–424.

22.Tomren MA., Másson M., Loftsson T., Tønnesen HH. Studies on curcumin and curcuminoids XXXI. Symmetric and asymmetric curcuminoids: stability, activity and complexation with cyclodextrin. Int J Pharm. 2007. 338(1-2):27–34.
23.Desai MP., Labhasetwar V., Amidon GL., Levy RJ. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm Res. 1996. 13(12):1838–1845.
24.Markovits N., Ben Amotz A., Levy Y. The effect of tomato-derived lycopene on low carotenoids and enhanced systemic inflammation and oxidation in severe obesity. Isr Med Assoc J. 2009. 11(10):598–601.
25.Kundu JK., Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008. 659(1-2):15–30.

26.TerzićJ. Grivennikov S., Karin E., Karin M. Inflammation and colon cancer. Gastroenterology. 2010. 138(6):2101–2114. .e5.

27.Baker RG., Hayden MS., Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011. 13(1):11–22.
28.Anisowicz A., Messineo M., Lee SW., Sager R. An NF-kappa B-like transcription factor mediates IL-1/TNF-alpha induction of gro in human fibroblasts. J Immunol. 1991. 147(2):520–527.
29.Jones E., Adcock IM., Ahmed BY., Punchard NA. Modulation of LPS stimulated NF-kappaB mediated Nitric Oxide production by PKCepsilon and JAK2 in RAW macrophages. J Inflamm (Lond). 2007. 4(1):23.

30.Ambs S., Hussain SP., Harris CC. Interactive effects of nitric oxide and the p53 tumor suppressor gene in carcinogenesis and tumor progression. FASEB J. 1997. 11(6):443–448.

31.Bogdan C., Röllinghoff M., Diefenbach A. The role of nitric oxide in innate immunity. Immunol Rev. 2000. 173(1):17–26.

32.Dubois RN., Abramson SB., Crofford L., Gupta RA., Simon LS., Van De Putte LB., Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998. 12(12):1063–1073.

33.Yang C., Liu X., Cao Q., Liang Q., Qiu X. Prostaglandin E receptors as inflammatory therapeutic targets for atherosclerosis. Life Sci. 2011. 88(5-6):201–205.

34.Chan G., Boyle JO., Yang EK., Zhang F., Sacks PG., Shah JP., Edelstein D., Soslow RA., Koki AT., Woerner BM., Masferrer JL., Dan-nenberg AJ. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999. 59(5):991–994.
35.Conner EM., Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996. 12(4):274–277.

36.Lee CW., Yen FL., Huang HW., Wu TH., Ko HH., Tzeng WS., Lin CC. Resveratrol nanoparticle system improves dissolution properties and enhances the hepatoprotective effect of resveratrol through antioxidant and anti-inflammatory pathways. J Agric Food Chem. 2012. 60(18):4662–4771.

37.Crispen PL., Uzzo RG., Golovine K., Makhov P., Pollack A., Horwitz EM., Greenberg RE., Kolenko VM. Vitamin E succinate inhibits NF-kappaB and prevents the development of a metastatic phenotype in prostate cancer cells: implications for chemoprevention. Prostate. 2007. 67(6):582–590.
38.Levy J., Bosin E., Feldman B., Giat Y., Miinster A., Danilenko M., Sharoni Y. Lycopene is a more potent inhibitor of human cancer cell proliferation than either alpha-carotene or beta-carotene. Nutr Cancer. 1995. 24(3):257–266.
39.Pastori M., Pfander H., Boscoboinik D., Azzi A. Lycopene in association with alpha-tocopherol inhibits at physiological concentrations proliferation of prostate carcinoma cells. Biochem Biophys Res Commun. 1998. 250(3):582–585.
40.Sahu A., Bora U., Kasoju N., Goswami P. Synthesis of novel biodegradable and self-assembling methoxy poly(ethylene glycol)-palmitate nanocarrier for curcumin delivery to cancer cells. Acta Biomater. 2008. 4(6):1752–1761.

41.Jeong HS., Han JG., Ha JH., Kim Y., Oh SH., Kim SS., Jeong MH., Choi GP., Park UY. Antioxidant activities and skin-whitening effects of nano-encapsuled water extract from rubus coreanus miquel. Korean J Med Crop Sci. 2009. 17(2):83–89.
Fig. 1.
Effect of FL or NFL on cell proliferation in Raw264.7 cells. FL: free lycopene, NEL: nanoencapsulated lycopene. Values not sharing the same purperscript letter are statistically different by Duncan's multiple range test in the same type of lycopene treatment (p < 0.05).
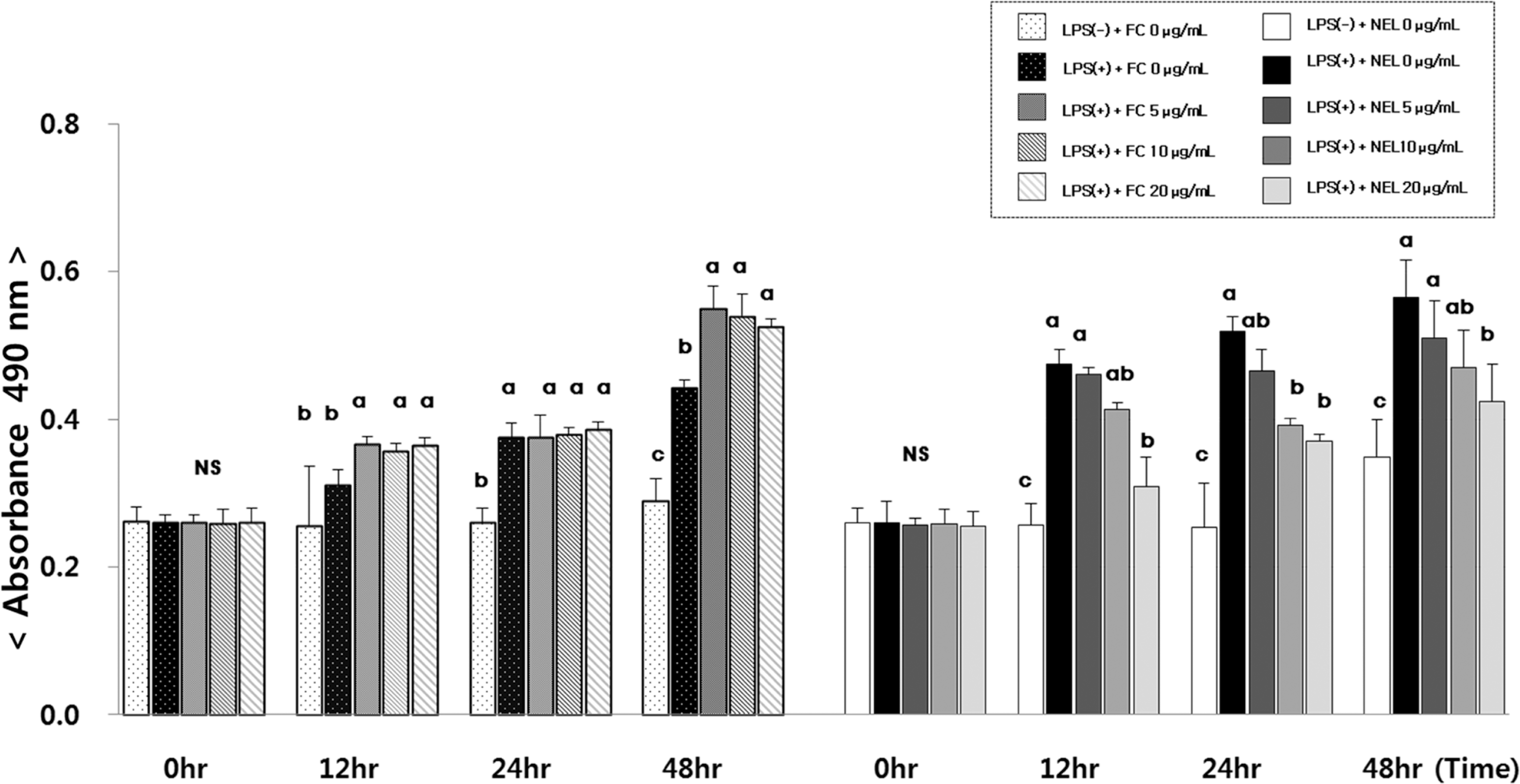
Fig. 2.
Effect of FL or NFL on IL-6 mRNA experssion and amount in Raw264.7 cells. FL: free lycopene, NEL: nanoencapsulated lycopene. Values not sharing the same purperscript letter are statistically different by Duncan's multiple range test in the same type of lycopene treatment (p < 0.05).
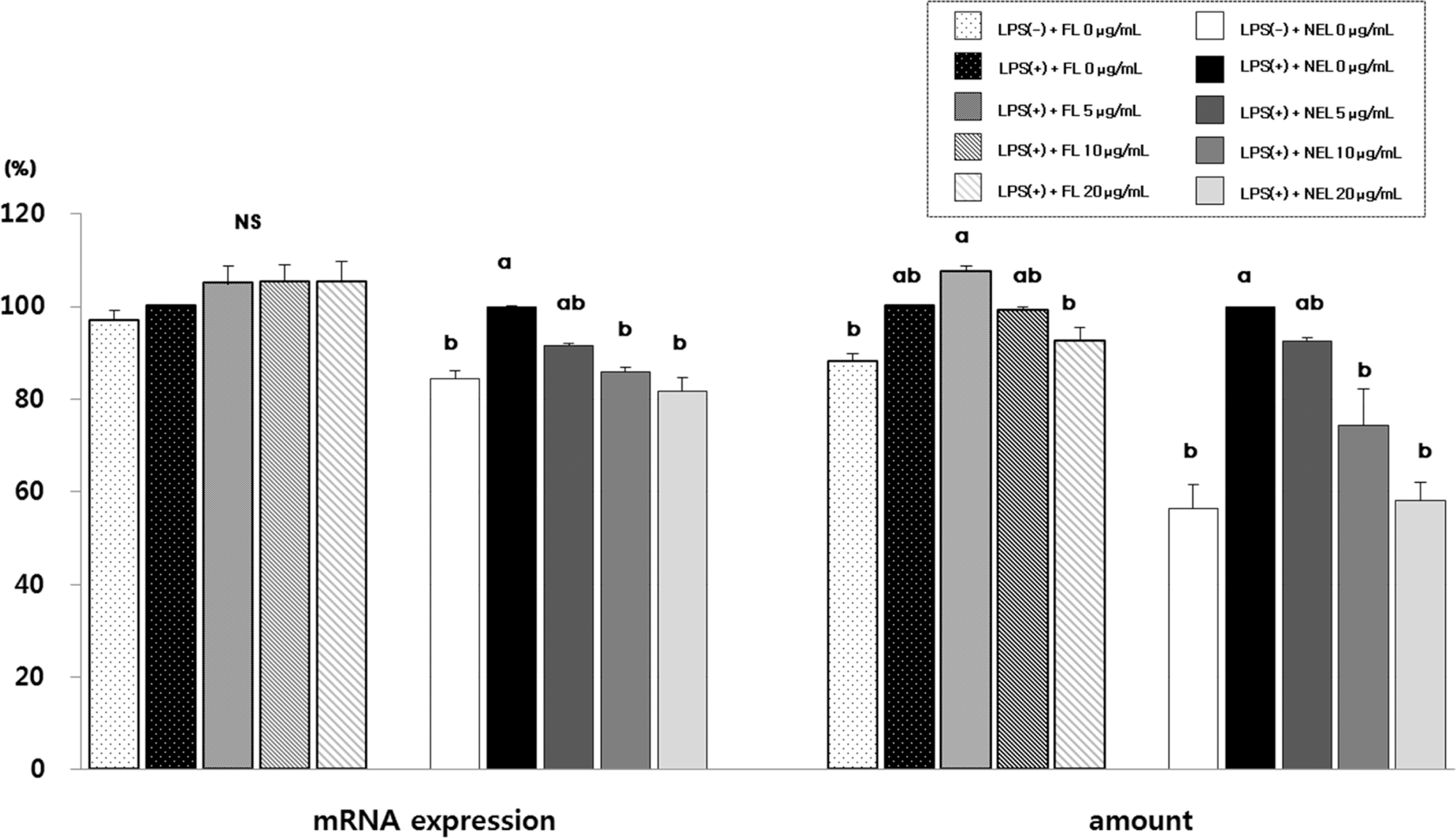
Fig. 3.
Effect of FL or NFL on IL-1β mRNA experssion and amount in Raw264.7 cells. FL: free lycopene, NEL: nanoencapsulated lycopene. Values not sharing the same purperscript letter are statistically different by Duncan's multiple range test in the same type of lycopene treatment (p < 0.05).
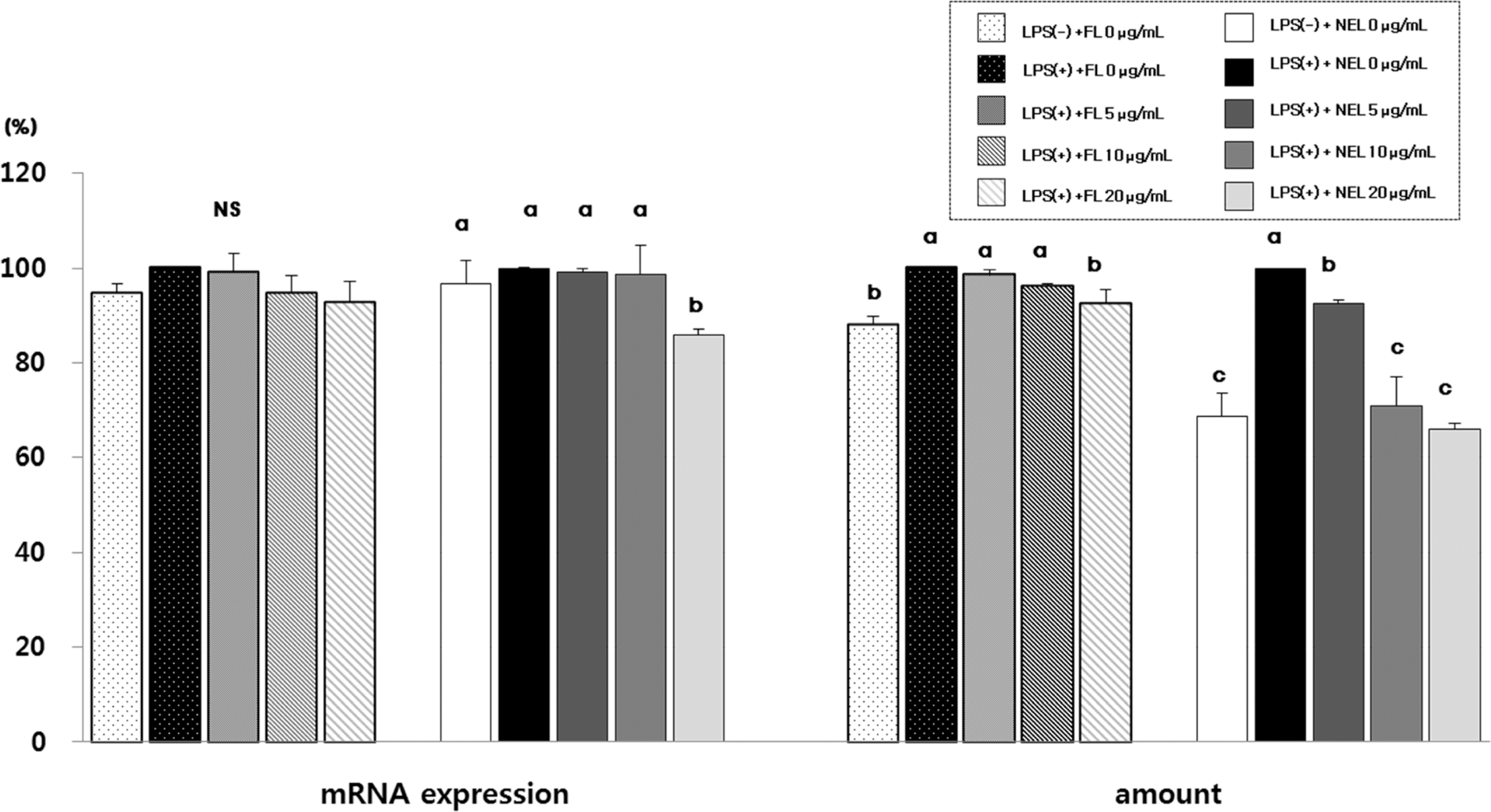
Fig. 4.
Effect of FL or NFL on TNF-α mRNA experssion and amount in Raw264.7 cells. FL: free lycopene, NEL: nanoencapsulated lycopene. Values not sharing the same purperscript letter are statistically different by Duncan's multiple range test in the same type of lycopene treatment (p < 0.05).
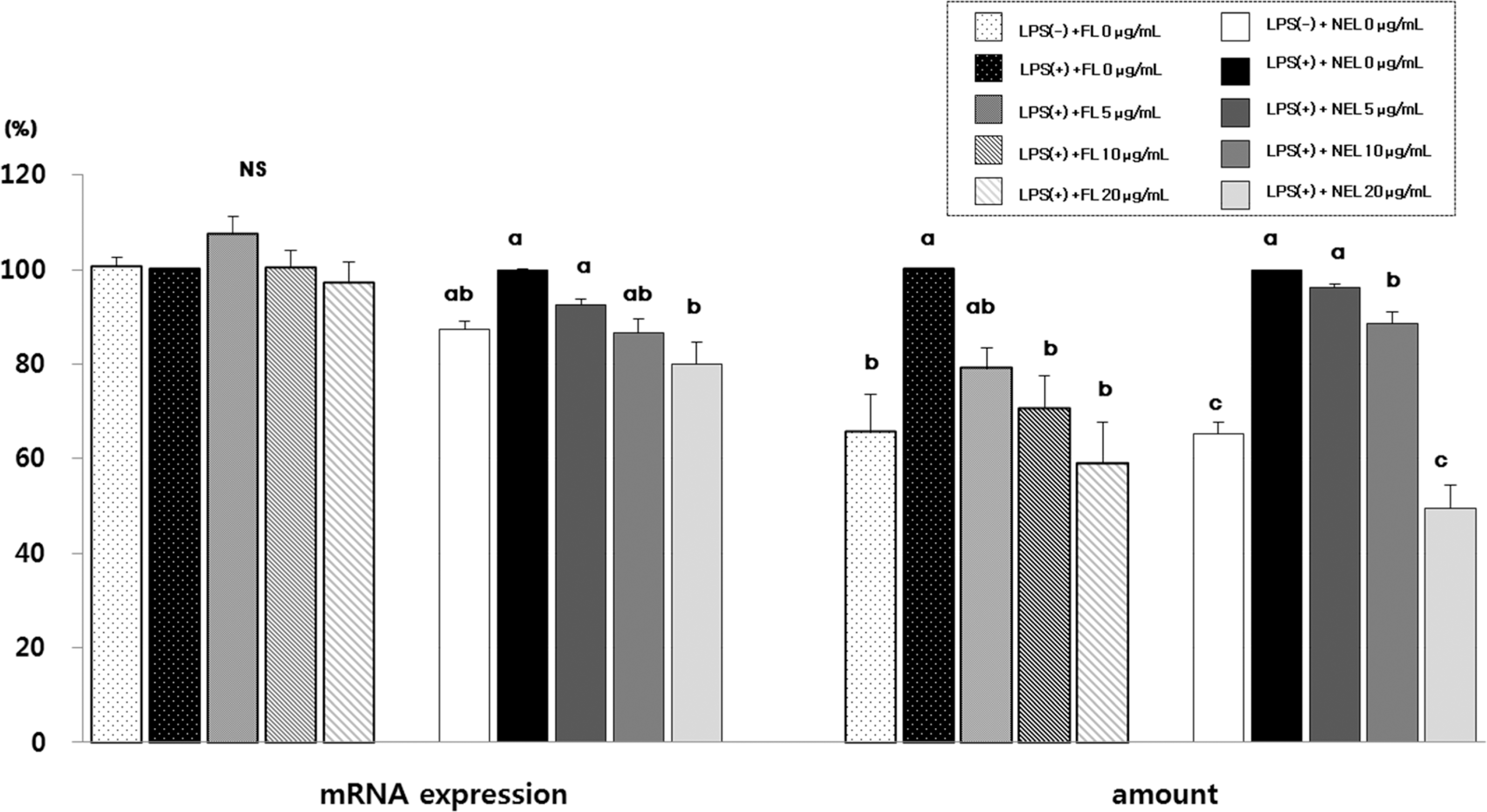
Fig. 5.
Effect of FL or NFL on iNOS and COX-2 mRNA experssion and amount in Raw264.7 cells. FL: free lycopene, NEL: nanoencapsulated lycopene. Values not sharing the same purperscript letter are statistically different by Duncan's multiple range test in the same type of lycopene treatment (p < 0.05).
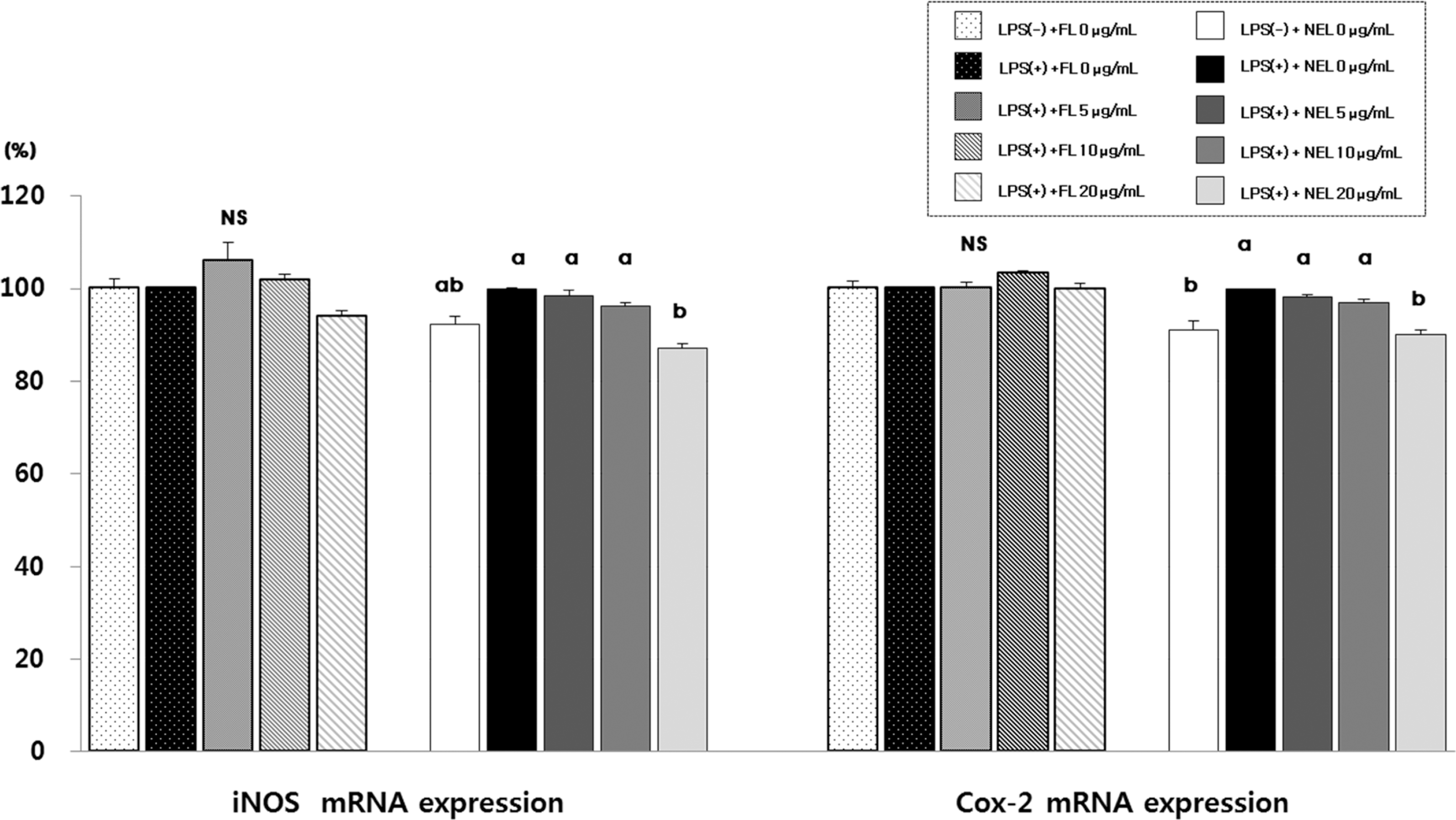
Table 1.
Primer sequences used for Real time PCR




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download