Abstract
Purpose:
In the present study, we aimed to evaluate the effect of sucrose containing 2 different levels of xylooligosaccharide on the glycemic index (GI) and blood glucose response in healthy adults.
Methods:
Healthy adults (4 male participants and 6 female participants, n = 10) were randomized to receive glucose, sucrose, sucrose containing 7% xylooligosaccharide active elements (Xylo 7), or sucrose containing 10% xylooligosaccharide active elements (Xylo 10). Each participant was administrated one of these materials once a week for 8 weeks and an oral glucose tolerance test was performed.
Results:
We found a reduction in the glycemic response to sucrose that included xylooligosaccharide active elements (Xylo 7 and Xylo 10). The glycemic indices of sucrose, Xylo 7 and Xylo 10 were 68.9, 54.7, and 52.5, respectively. The GI values of Xylo 7 and Xylo 10 were similar to that of foods with low GI. The percentage reduction of GI value caused by sucrose containing xylooligosaccharide active elements was significantly different and dose-dependent as compared to that caused by sucrose alone (p < 0.05). The reduction in the glycemic response to Xylo 7 and Xylo 10 was 21% and 24%, respectively, as compared to the glycemic response to sucrose. The attenuation of the glycemic response to Xylo 10 tended to be higher than that for Xylo 7 when the percentage of body fat was increased.
Go to : 
REFERENCES
1.Bray GA., Nielsen SJ., Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004. 79(4):537–543.

2.Korea Rural Economic Institute. Food balance sheet 2012. Seoul: Korea Rural Economic Institute;2013.
3.Song S., Lee JE., Song WO., Paik HY., Song Y. Carbohydrate intake and refined-grain consumption are associated with metabolic syndrome in the Korean adult population. J Acad Nutr Diet. 2014. 114(1):54–62.

4.Shin HL. Consumer attitude survey: beverage purchasing behaviors and preference [dissertation]. Seoul: Sejong University;2010.
5.Latulippe ME., Skoog SM. Fructose malabsorption and intolerance: effects of fructose with and without simultaneous glucose ingestion. Crit Rev Food Sci Nutr. 2011. 51(7):583–592.

6.Chen MS., Kao CS., Chang CJ., Wu TJ., Fu CC., Chen CJ., Tai TY. Prevalence and risk factors of diabetic retinopathy among noninsu-lin-dependent diabetic subjects. Am J Ophthalmol. 1992. 114(6):723–730.

7.Malik VS., Popkin BM., Bray GA., Després JP., Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010. 121(11):1356–1364.

8.Yoo H., Kim Y. A study on the characteristics of nutrient intake in metabolic syndrome subjects. Korean J Nutr. 2008. 41(6):510–517.
9.Colditz GA., Manson JE., Stampfer MJ., Rosner B., Willett WC., Speizer FE. Diet and risk of clinical diabetes in women. Am J Clin Nutr. 1992. 55(5):1018–1023.

10.de Koning L., Malik VS., Rimm EB., Willett WC., Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr. 2011. 93(6):1321–1327.

11.Salmerón J., Manson JE., Stampfer MJ., Colditz GA., Wing AL., Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997. 277(6):472–477.

12.Dallongeville J., Charbonnel B., Desprès JP. Sugar-sweetened beverages and cardiometabolic risk. Presse Med. 2011. 40(10):910–915.
13.Alonso S., Setser C. Functional replacements for sugars in foods. Trends Food Sci Technol. 1994. 5(5):139–146.

14.Fiordaliso M., Kok N., Desager JP., Goethals F., Deboyser D., Rober-froid M., Delzenne N. Dietary oligofructose lowers triglycerides, phospholipids and cholesterol in serum and very low density lipoproteins of rats. Lipids. 1995. 30(2):163–167.

15.Lee OS., Rhee IK. The production of xylooligosaccharides with microbial xylanase. Food Ind Nutr. 2001. 6(1):21–24.
16.Akpinar O., Erdogan K., Bostanci S. Enzymatic production of xylo-oligosaccharide from selected agricultural wastes. Food Bioprod Process. 2009. 87(2):145–151.

17.Hsu CK., Liao JW., Chung YC., Hsieh CP., Chan YC. Xylooligosac-charides and fructooligosaccharides affect the intestinal microbiota and precancerous colonic lesion development in rats. J Nutr. 2004. 134(6):1523–1528.

18.Moon SH., Lee KS., Kyung MG., Jung SW., Park YJ., Yang CK. Study on the proper D-xylo concentration in sugar mixture to reduce glycemic index (GI) value in the human clinical model. Korean J Food Nutr. 2012. 25(4):787–792.
19.Kyung M., Choe H., Jung S., Lee K., Jo S., Seo S., Choe K., Yang CK., Yoo SH., Kim Y. Effects of xylooligosaccharide-sugar mixture on glycemic index (GI) and blood glucose response in healthy adults. J Nutr Health. 2014. 47(4):229–235.

20.Jenkins DJ., Wolever TM., Taylor RH., Barker H., Fielden H., Baldwin JM., Bowling AC., Newman HC., Jenkins AL., Goff DV. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981. 34(3):362–366.

21.Willett W., Manson J., Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Am J Clin Nutr. 2002. 76(1):274S–280S.

22.Brand-Miller JC., Holt SH., Pawlak DB., McMillan J. Glycemic index and obesity. Am J Clin Nutr. 2002. 76(1):281S–285S.

23.Wolever TM., Vorster HH., Björck I., Brand-Miller J., Brighenti F., Mann JI., Ramdath DD., Granfeldt Y., Holt S., Perry TL., Venter C., Wu Xiaomei. Determination of the glycaemic index of foods: interlaboratory study. Eur J Clin Nutr. 2003. 57(3):475–482.

24.Brouns F., Bjorck I., Frayn KN., Gibbs AL., Lang V., Slama G., Wolever TM. Glycaemic index methodology. Nutr Res Rev. 2005. 18(1):145–171.

25.Foster-Powell K., Holt SH., Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002. 76(1):5–56.

26.Lee K., Moon S., Jung S., Park YJ., Yoon S., Choe K., Yang C. Glycemic index of sucrose with D-xylose (XF) in humans. Curr Top Nutraceutical Res. 2013. 11(1/2):35–40.
27.Venter CS., Vorster HH., Cummings JH. Effects of dietary propionate on carbohydrate and lipid metabolism in healthy volunteers. Am J Gastroenterol. 1990. 85(5):549–553.
28.Lecerf JM., Dépeint F., Clerc E., Dugenet Y., Niamba CN., Rhazi L., Cayzeele A., Abdelnour G., Jaruga A., Younes H., Jacobs H., Lam-brey G., Abdelnour AM., Pouillart PR. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br J Nutr. 2012. 108(10):1847–1858.

29.Rodríguez-Cabezas ME., Camuesco D., Arribas B., Garrido-Mesa N., Comalada M., Bailón E., Cueto-Sola M., Utrilla P., Guerra-Hernández E., Pérez-Roca C., Gálvez J., Zarzuelo A. The combination of fructooligosaccharides and resistant starch shows prebiotic additive effects in rats. Clin Nutr. 2010. 29(6):832–839.

30.Flickinger EA., Wolf BW., Garleb KA., Chow J., Leyer GJ., Johns PW., Fahey GC Jr. Glucose-based oligosaccharides exhibit different in vitro fermentation patterns and affect in vivo apparent nutrient digestibility and microbial populations in dogs. J Nutr. 2000. 130(5):1267–1273.

31.Younes H., Coudray C., Bellanger J., Demigné C., Rayssiguier Y., Rémésy C. Effects of two fermentable carbohydrates (inulin and resistant starch) and their combination on calcium and magnesium balance in rats. Br J Nutr. 2001. 86(4):479–485.

32.Seri K., Sanai K., Matsuo N., Kawakubo K., Xue C., Inoue S. L-arabinose selectively inhibits intestinal sucrase in an uncompetitive manner and suppresses glycemic response after sucrose ingestion in animals. Metabolism. 1996. 45(11):1368–1374.

33.Bae YJ., Bak YK., Kim B., Kim MS., Lee JH., Sung MK. Coconut-derived D-xylose affects postprandial glucose and insulin responses in healthy individuals. Nutr Res Pract. 2011. 5(6):533–539.

34.Alles MS., de Roos NM., Bakx JC., van de Lisdonk E., Zock PL., Hautvast GA. Consumption of fructooligosaccharides does not favorably affect blood glucose and serum lipid concentrations in patients with type 2 diabetes. Am J Clin Nutr. 1999. 69(1):64–69.

35.Sheu WH., Lee IT., Chen W., Chan YC. Effects of xylooligosaccha-rides in type 2 diabetes mellitus. J Nutr Sci Vitaminol (Tokyo). 2008. 54(5):396–401.

36.Chung YC., Hsu CK., Ko CY., Chan YC. Dietary intake of xylooli-gosaccharides improves the intestinal microbiota, fecal moisture, and pH value in the elderly. Nutr Res. 2007. 27(12):756–761.

37.Gobinath D., Madhu AN., Prashant G., Srinivasan K., Prapulla SG. Beneficial effect of xylo-oligosaccharides and fructooligosaccha-rides in streptozotocin-induced diabetic rats. Br J Nutr. 2010. 104(1):40–47.

38.Yang J., Summanen PH., Henning SM., Hsu M., Lam H., Huang J., Tseng CH., Dowd SE., Finegold SM., Heber D., Li Z. Xylooligosac-charide supplementation alters gut bacteria in both healthy and prediabetic adults: a pilot study. Front Physiol. 2015. 6:216.

39.Ebbeling CB., Ludwig DS. Treating obesity in youth: should dietary glycemic load be a consideration? Adv Pediatr. 2001. 48:179–212.
40.Murakami K., Sasaki S., Takahashi Y., Okubo H., Hosoi Y., Horiguchi H., Oguma E., Kayama F. Dietary glycemic index and load in relation to metabolic risk factors in Japanese female farmers with traditional dietary habits. Am J Clin Nutr. 2006. 83(5):1161–1169.

41.Salmerón J., Manson JE., Stampfer MJ., Colditz GA., Wing AL., Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997. 277(6):472–477.

42.Wolever TM., Jenkins DJ., Vuksan V., Jenkins AL., Wong GS., Josse RG. Beneficial effect of low-glycemic index diet in overweight NIDDM subjects. Diabetes Care. 1992. 15(4):562–564.

43.Joo GJ., Rhee IK., Kim SO., Rhee SJ. Effect of dietary xylooligosac-charide on indigestion and retarding effect of bile acid movement across a dialysis membrane. J Korean Soc Food Sci Nutr. 1998. 27(4):705–711.
Go to : 
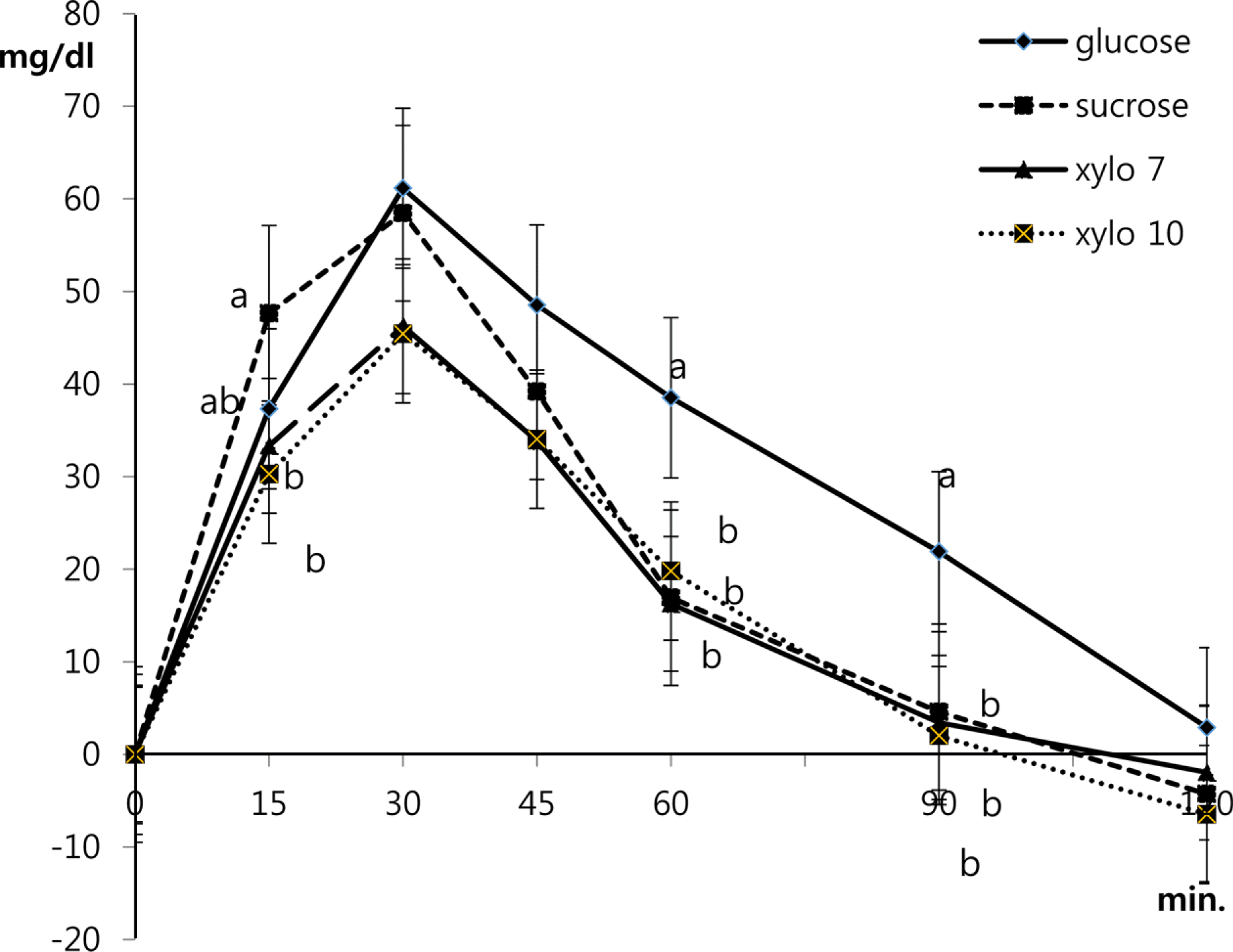 | Fig. 1.Mean blood glucose responses after administration of control food (glucose) and test food (sucrose, Xylo 7 and Xylo 10). Each value is the mean ± SD. Different alphabets at same time are significant (p < 0.05) between groups. Xylo 7: sucrose with 14% xylooligosaccaride powder (active element X2~X7 7%), Xylo 10: sucrose with 20% xylooligosaccaride powder (active element X2~X7 10%) |
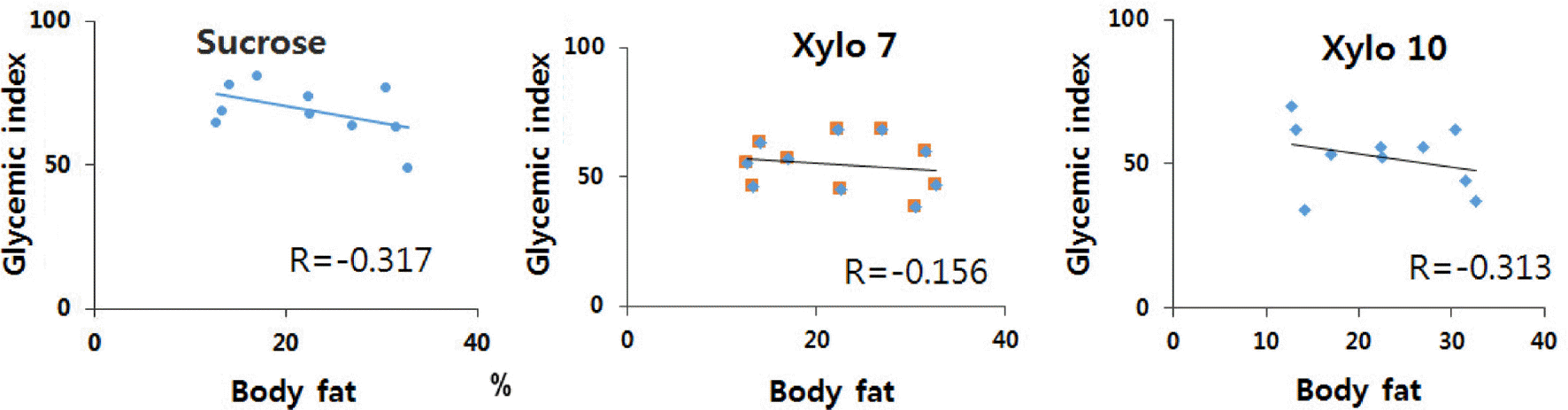 | Fig. 2.Correlation between percent body fat and glycemic index of Xylo 7 and Xylo 10. Xylo 7: sucrose with 14% xylooligosaccaride powder (active element X2~X7 7%), Xylo 10: sucrose with 20% xylooligosaccaride powder (active element X2~X7 10%) |
Table 1.
Test food composition in the clinical trial
Table 2.
Baseline characteristics of the subjects in the clinical trial (n = 10)
Table 3.
Glycemic indices of Xylo 7 and Xylo 10 (n = 10)
| Variables | Sucrose | Xylo 74) | Xylo 105) |
|---|---|---|---|
| Gl | 68.9 ± 9.41)a2) | 54.7 ± 10.3b | 52.5 ± 11.3b |
| CV3) | 13.60 | 18.85 | 21.51 |
Table 4.
The changes in blood glucose variables (n = 10)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download