Abstract
Purpose
In this study, we investigated the effects of jaceosidin on blood glucose regulation in type 1 diabetic mice. Methods: C57BL/6 mice were divided into four groups; normal control (Normal), diabetes control (D-Control), diabetes low-jaceosidin (D-0.005%), and diabetes high-jaceosidin (D-0.02%). Type 1 diabetes was induced by streptozotocin and mice were then fed a diet containing jaceosidin for eight weeks. Fasting blood glucose, oral glucose tolerance test, insulin tolerance test, lipid peroxidation, and antioxidant enzyme activities were assessed. Results: Jaceosidin supplementation for eight weeks had no effect on body weight, organ weight, and blood lipid profiles. However, jaceosidin supplementation significantly lowered fasting blood glucose level and reduced insulin resistance. We also found that jaceosidin supplementation increased antioxidant capacity by enhancement of catalase and GSH-px activities. Conclusion: These results suggest that jaceosidin could be a therapeutic candidate to ameliorate hyperglycemia through increase of antioxidant enzyme activity.
Go to : 
References
1. Lim S, Lee EJ, Koo BK, Cho SI, Park KS, Jang HC, Kim SY, Lee HK. Increasing trends of metabolic syndrome in Korea-based on Korean National Health and Nutrition Examination Surveys. J Korean Diabetes Assoc. 2005; 29(5):432–439.
2. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998; 15(7):539–553.

3. Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006; 55(5):1463–1469.
4. Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991; 40(4):405–412.

5. Eppens MC, Craig ME, Cusumano J, Hing S, Chan AK, Howard NJ, Silink M, Donaghue KC. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care. 2006; 29(6):1300–1306.

6. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001; 414(6865):813–820.

7. Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonne-veld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004; 53(1):195–199.
8. Giacco F, Brownlee M. Chapter 35. Pathogenesis of microvascular complications. Holt RI, Cockram C, Flyvbjerg A, Goldstein BJ, editors. editors.Textbook of Diabetes. 4th edition.Chichester: Wiley-Blackwell;2010. p. 555.
10. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002; 23(5):599–622.

11. Yasunari K, Maeda K, Nakamura M, Yoshikawa J. Oxidative stress in leukocytes is a possible link between blood pressure, blood glucose, and C-reacting protein. Hypertension. 2002; 39(3):777–780.

12. Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006; 295(14):1681–1687.

13. Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999; 48(1):1–9.

14. Irshad M, Chaudhuri PS. Oxidant-antioxidant system: role and significance in human body. Indian J Exp Biol. 2002; 40(11):1233–1239.
15. Lee SD, Park HH, Kim DW. Bang BB. Bioactive constituents and utilities of Artemisia sp. as medical herb and foodstuff. Korean J Food Nutr. 2000; 13(5):490–505.
16. Ryu SN, Kang SS, Kim JS, Ku BI. Quantitative analysis of eupatilin and Jaceosidin in Artemisia herba. Korean J Crop Sci. 2004; 49(6):452–456.
17. Al-Mustafa AH, Al-Thunibat OY. Antioxidant activity of some Jordanian medicinal plants used traditionally for treatment of diabetes. Pak J Biol Sci. 2008; 11(3):351–358.

18. Hill JO, Peters JC. Biomarkers and functional foods for obesity and diabetes. Br J Nutr. 2002; 88(Suppl 2):S213–S218.

19. Min SW, Kim NJ, Baek NI, Kim DH. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps on carrageenan-induced inflammation in mice. J Ethnopharmacol. 2009; 125(3):497–500.

20. Riccardi G, Capaldo B, Vaccaro O. Functional foods in the management of obesity and type 2 diabetes. Curr Opin Clin Nutr Metab Care. 2005; 8(6):630–635.

21. Kang YJ. Beneficial effects of eupatilin and jaceosidin isolated from Artemisia princeps on regulation of glucose, lipid and antioxidant metabolism in type 2 diabetic mice [dissertation]. Daegu: Kyungpook National University;2008.
22. Farhangkhoee H, Khan ZA, Mukherjee S, Cukiernik M, Barbin YP, Karmazyn M, Chakrabarti S. Heme oxygenase in diabetes-induced oxidative stress in the heart. J Mol Cell Cardiol. 2003; 35(12):1439–1448.

23. Nagareddy PR, Xia Z, McNeill JH, MacLeod KM. Increased expression of iNOS is associated with endothelial dysfunction and impaired pressor responsiveness in streptozotocin-induced diabetes. Am J Physiol Heart Circ Physiol. 2005; 289(5):H2144–H2152.

24. Komers R, Lindsley JN, Oyama TT, Schutzer WE, Reed JF, Mader SL, Anderson S. Immunohistochemical and functional correlations of renal cyclooxygenase-2 in experimental diabetes. J Clin Invest. 2001; 107(7):889–898.

25. Huang X, Yuang J, Goddard A, Foulis A, James RF, Lernmark A, Pujol-Borrell R, Rabinovitch A, Somoza N, Stewart TA. Interferon expression in the pancreases of patients with type I diabetes. Diabetes. 1995; 44(6):658–664.

26. Wentholt IM, Kulik W, Michels RP, Hoekstra JB, DeVries JH. Glucose fluctuations and activation of oxidative stress in patients with type 1 diabetes. Diabetologia. 2008; 51(1):183–190.

27. Ruggenenti P, Remuzzi G. Primary prevention of renal failure in diabetic patients: the Bergamo Nephrologic Diabetes Complication Trial. J Hypertens Suppl. 1998; 16(1):S95–S97.
28. Blake R, Trounce IA. Mitochondrial dysfunction and complications associated with diabetes. Biochim Biophys Acta. 2014; 1840(4):1404–1412.

29. Yang HG, Kim HJ, Kim HS, Park SN. Antioxidative and antibacterial activities of Artemisia princeps Pampanini extracts. Korean J Microbiol Biotechnol. 2012; 40(3):250–260.

30. Kim MJ, Kim DH, Lee KW, Yoon DY, Surh YJ. Jaceosidin induces apoptosis in ras-transformed human breast epithelial cells through generation of reactive oxygen species. Ann N Y Acad Sci. 2007; 1095:483–495.

31. Lortz S, Tiedge M. Sequential inactivation of reactive oxygen species by combined overexpression of SOD isoforms and catalase in insulin-producing cells. Free Radic Biol Med. 2003; 34(6):683–688.

32. Fu Z, Zhen W, Yuskavage J, Liu D. Epigallocatechin gallate delays the onset of type 1 diabetes in spontaneous nonobese diabetic mice. Br J Nutr. 2011; 105(8):1218–1225.

33. Alam MM, Meerza D, Naseem I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014; 109(1):8–14.

34. Hong JH, Jeon JL, Lee JH, Lee IS. Antioxidative properties of Artemisia princeps Pamp. J Korean Soc Food Sci Nutr. 2007; 36(6):657–662.

35. Lee SJ, Shin JH, Ju JC, Kang SK, Sung NJ. Hypoglycemic and hypolipidemic effects of Orostachys japonicus with medicinal herbs in streptozotocin-induced diabetic rats. J Korean Soc Food Sci Nutr. 2013; 42(4):587–594.

36. Ramachandran V, Saravanan R. Asiatic acid prevents lipid peroxidation and improves antioxidant status in rats with streptozotocin-induced diabetes. J Funct Foods. 2013; 5(3):1077–1087.

37. Kim MW. Effect of Sea Buckthorn leaves on hepatic enzyme levels in streptozotocin induced diabetic rats. J Korean Soc Food Sci Nutr. 2013; 42(1):40–45.

38. Anaya-Eugenio GD, Rivero-Cruz I, Rivera-Chávez J, Mata R. Hypoglycemic properties of some preparations and compounds from Artemisia ludoviciana Nutt. J Ethnopharmacol. 2014; 155(1):416–425.

39. Yuan H, Meng S, Wang G, Gong Z, Sun W, He G. Hypoglycemic effect of triterpenoid-rich extracts from Euryale ferox shell on normal and streptozotocin-diabetic mice. Pak J Pharm Sci. 2014; 27(4):859–864.
Go to : 
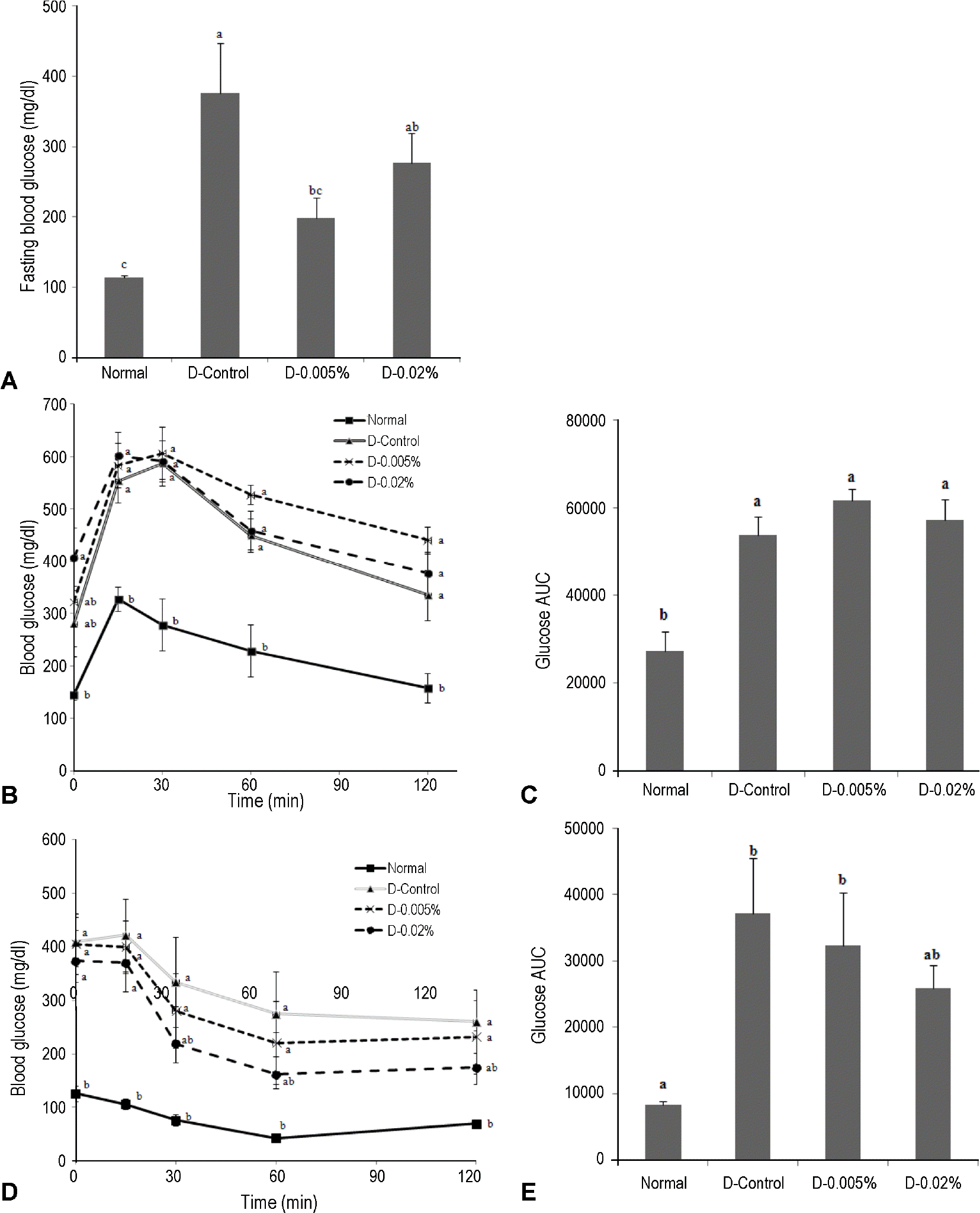 | Fig. 1.Effects of jaceosidin intake on fasting blood glucose, oral glucose tolerance test and insulin tolerance test in STZ-induced mice. A: Fasting blood glucose levels after 8 weeks of diet, B: Oral glucose tolerance test (OGTT) was performed at 6 week of diet. C: Area of under curve (AUC) was calculated based on OGTT results. D: Insulin tolerance test (ITT) was performed at 7 week of diet. E: AUC was calculated based on ITT results. |
Table 1.
Composition of experimental diets (g/Kg diet)
Table 2.
Effects of jaceosidin on changes in body weight, food intake, and food efficiency ratio in STZ-induced diabetic mice
Table 3.
Effects of jaceosidin on organ weight in STZ-induced diabetic mice
Table 4.
Effects of jaceosidin on liver function in STZ-induced diabetic mice
| Normal | D-Control | D-0.005% | D-0.02% | |
|---|---|---|---|---|
| GOT (U/L) | 65.8 ± 8.91) | 131.8 ± 66.6 | 93.6 ± 18.1 | 132.0 ± 70.7 |
| GPT (U/L) | 16.6 ± 3.3 | 55.2 ± 36.2 | 32.4 ± 5.4 | 48.7 ± 40.5 |
Table 5.
Effects of jaceosidin on liver lipid peroxidation and antioxidant enzyme activities in STZ-induced diabetic mice




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download