Abstract
Purpose
Previous studies have shown that treatment with Smilax china L. leaf extract (SCLE) produces antidiabetic effects due to α-glucosidase inhibition. In this study, we examined the mechanism underlying these antidiabetic effects by examining glucose uptake in HepG2 cells cultured with SCLE. Methods: Glucose uptake and glucokinase activity were examined using an assay kit. Expression of glucose transporter (GLUT)-2, GLUT-4, and HNF-1α was measured by RT-PCR or western blot. Results: Treatment with SCLE resulted in enhanced glucose uptake in HepG2 cells, and this effect was especially pronounced when cells were cultured in an insulin-free medium. SCLE induced an increase in expression of GLUT-2 but not GLUT-4. The increase in the levels of HNF-1α, a GLUT-2 transcription factor, in total protein extract and nuclear fraction suggest that the effects of SCLE may occur at the level of GLUT-2 transcription. In addition, by measuring the change in glucokinase activity following SCLE treatment, we confirmed that SCLE stimulates glucose utilization by direct activation of this enzyme. Conclusion: These results demonstrate that the potential antidiabetic activity of SCLE is due at least in part to stimulation of glucose uptake and an increase in glucokinase activity, and that SCLE-stimulated glucose uptake is mediated through enhancement of GLUT-2 expression by inducing expression of its transcription factor, HNF-1α.
Go to : 
References
1. Ko YJ, Kim JY, Kim EJ, Kim EJ, Seol HG, Park GH, Chung GY, Ryu CH. Treatment of Smilax china L. root extract for improvement of storage stability of Mang-gae rice cake. Korean J Food Preserv. 2012; 19(2):167–172.

2. Kang HS, You HC, Choi Y, Kim HK, Jo SM, Yoon BJ. Effect of Smilax china L. Rhizome extract on heavy metal contents in rats. Korean J Food Nutr. 2011; 24(2):233–238.

3. Cha BC, Lee EH. Antioxidant activities of flavonoids from the leaves of Smilax china linne. Korean J Pharmacogn. 2007; 38(1):31–36.
4. Kim KK, Kang YH, Kim DJ, Kim TW, Choe M. Comparison of antioxidant, α-glucosidase inhibition and antiinflammatory activities of the leaf and root extracts of Smilax china L. J Nutr Health. 2013; 46(4):315–323.
5. Bhati R, Singh A, Saharan VA, Ram V, Bhandari A. Pharmacognostical standardization, extraction and anti-diabetic activity of Smilax china L. rhizome. Asian J Tradit Med. 2011; 6(5):218–223.
6. Kim DH, Park SC, Lee JH, Lee HY, Cho MK, Choi JY, Kim SY, Park SH. Recent research trends in Korean medicine treatment of diabetes mellitus – Focusing on domestic articles from 2008 to 2013 –. J Korean Orient Intern Med. 2013; 34(3):240–255.
7. Lee DS, Jeong GS, An RB, Li B, Byun E, Kim YC. Search for plant extracts with protective effects of pancreatic beta cell against oxidative stress. Korean J Pharmacogn. 2008; 39(4):335–340.
8. Song S, Paik HY, Song Y. The relationship between intake of nutrients and food groups and insulin resistance in Korean adults: using the Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV, 2007–2009). Korean J Nutr. 2013; 46(1):61–71.

9. Kumar S, Narwal S, Kumar V, Prakash O. α-Glucosidase inhibitors from plants: a natural approach to treat diabetes. Pharmacogn Rev. 2011; 5(9):19–29.

10. Choung ES, Bak JP, Choi H, Jang GS, Kang SH, Kang SC, Zee OP. Effects of antidiabetic and GLUT4 gene expression of Acanthopanax senticosus extracts. Korean J Pharmacogn. 2008; 39(3):228–232.
11. Moore MC, Coate KC, Winnick JJ, An Z, Cherrington AD. Regulation of hepatic glucose uptake and storage in vivo. Adv Nutr. 2012; 3(3):286–294.

12. Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes. 1999; 48(5):1198–1214.

13. Oosterveer MH, Schoonjans K. Hepatic glucose sensing and integrative pathways in the liver. Cell Mol Life Sci. 2014; 71(8):1453–1467.

14. Takanaga H, Chaudhuri B, Frommer WB. GLUT1 and GLUT9 as major contributors to glucose influx in HepG2 cells identified by a high sensitivity intramolecular FRET glucose sensor. Biochim Biophys Acta. 2008; 1778(4):1091–1099.

15. Kasai D, Adachi T, Deng L, Nagano-Fujii M, Sada K, Ikeda M, Kato N, Ide YH, Shoji I, Hotta H. HCV replication suppresses cellular glucose uptake through down-regulation of cell surface expression of glucose transporters. J Hepatol. 2009; 50(5):883–894.

16. Nistor Baldea LA, Martineau LC, Benhaddou-Andaloussi A, Arnason JT, Lévy É, Haddad PS. Inhibition of intestinal glucose absorption by anti-diabetic medicinal plants derived from the James Bay Cree traditional pharmacopeia. J Ethnopharmacol. 2010; 132(2):473–482.

17. Nordlie RC, Foster JD, Lange AJ. Regulation of glucose production by the liver. Annu Rev Nutr. 1999; 19:379–406.

18. Cerf ME. High fat diet modulation of glucose sensing in the beta-cell. Med Sci Monit. 2007; 13(1):RA12–RA17.
19. David-Silva A, Freitas HS, Okamoto MM, Sabino-Silva R, Schaan BD, Machado UF. Hepatocyte nuclear factors 1α/4α and forkhead box A2 regulate the solute carrier 2A2 (Slc2a2) gene expression in the liver and kidney of diabetic rats. Life Sci. 2013; 93(22):805–813.

20. Ban N, Yamada Y, Someya Y, Miyawaki K, Ihara Y, Hosokawa M, Toyokuni S, Tsuda K, Seino Y. Hepatocyte nuclear factor-1al-pha recruits the transcriptional coactivator p300 on the GLUT2 gene promoter. Diabetes. 2002; 51(5):1409–1418.
21. Kim JW, Ahn YH. CCAAT/enhancer binding protein regulates the promoter activity of the rat GLUT2 glucose transporter gene in liver cells. Biochem J. 1998; 336(Pt 1):83–90.

22. Stevens BD, Litchfield J, Pfefferkorn JA, Atkinson K, Perreault C, Amor P, Bahnck K, Berliner MA, Calloway J, Carlo A, Derksen DR, Filipski KJ, Gumkowski M, Jassal C, MacDougall M, Murphy B, Nkansah P, Pettersen J, Rotter C, Zhang Y. Discovery of an intravenous hepatoselective glucokinase activator for the treatment of inpatient hyperglycemia. Bioorg Med Chem Lett. 2013; 23(24):6588–6592.

23. Lloyd DJ, St Jean DJ Jr, Kurzeja RJ, Wahl RC, Michelsen K, Cupples R, Chen M, Wu J, Sivits G, Helmering J, Komorowski R, Ashton KS, Pennington LD, Fotsch C, Vazir M, Chen K, Chmait S, Zhang J, Liu L, Norman MH, Andrews KL, Bart-berger MD, Van G, Galbreath EJ, Vonderfecht SL, Wang M, Jordan SR, Véniant MM, Hale C. Antidiabetic effects of glucokinase regulatory protein small-molecule disruptors. Nature. 2013; 504(7480):437–440.

24. Kim HS, Kim TW, Kim DJ, Kim KK, Choe M. Effects of medicinal plant water extracts on expression of anti-diabetic enzymes mRNA. J Korean Soc Food Sci Nutr. 2013; 42(7):1008–1014.

25. Kim HS, Kim TW, Kim DJ, Lee JS, Choe M. Effects of medicinal herb water extracts on expression of hepatic glucokinase, pyruvate dehydrogenase and acetyl-CoA carboxylase mRNA. Korean J Nutr. 2013; 46(2):119–125.

26. Klip A, Tsakiridis T, Marette A, Ortiz PA. Regulation of expression of glucose transporters by glucose: a review of studies in vivo and in cell cultures. FASEB J. 1994; 8(1):43–53.

27. Párrizas M, Maestro MA, Boj SF, Paniagua A, Casamitjana R, Gomis R, Rivera F, Ferrer J. Hepatic nuclear factor 1-alpha directs nucleosomal hyperacetylation to its tissue-specific transcriptional targets. Mol Cell Biol. 2001; 21(9):3234–3243.
Go to : 
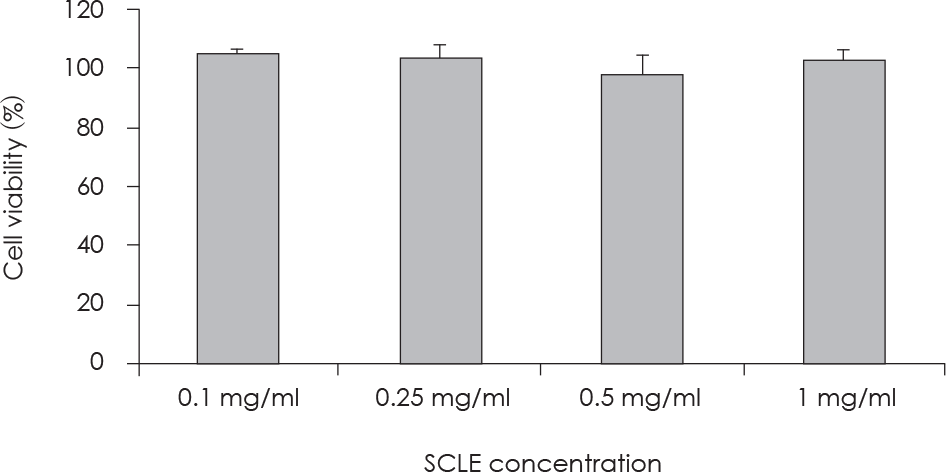 | Fig. 1.Concentration-dependent effects of SCLE on HepG2 cell growth. Cell viability was analyzed using the Cell Counting Kit-8 (CCK-8) assay kit. Each bar is the Mean ± SD, derived from three independent experiments. |
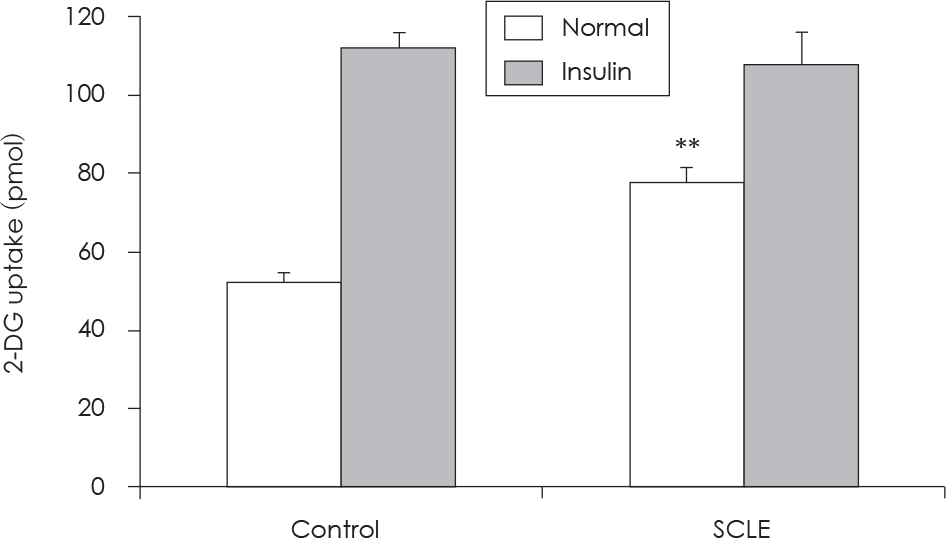 | Fig. 2.Induction of glucose uptake following Smilax china L. leaf extract (SCLE) treatment. HepG2 cells were incubated for 20 min in a KRPH buffer containing SCLE (0.25 mg/ml). The 2-deoxyglucose (2-DG) assay was performed 20 min later, as detailed in “Methods.” The 2DG uptake was expressed in pmol in normal and insulin conditions. Results are presented as the Mean ± SD of three independent experiments. ∗∗: p < 0.01, Normal control versus Normal + SCLE. |
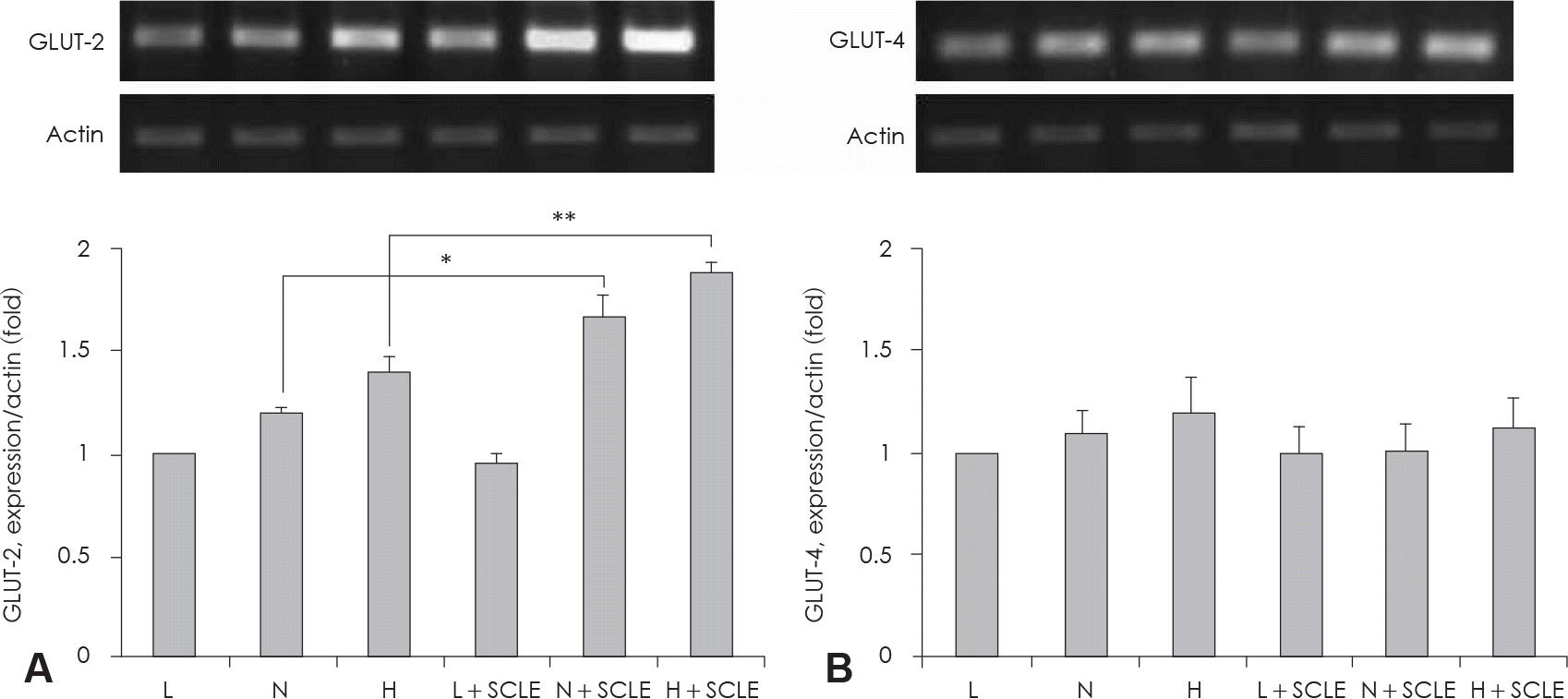 | Fig. 3.Measurement of GLUT-2, −4 mRNA expression. A: Effect of a Smilax china L. leaf extract (SCLE) treatment on GLUT-2 mRNA expression in HepG2 cells. B: Effect of SCLE treatment on GLUT-4 mRNA in HepG2 cells. Values are the Mean ± SD of triplicate determinations. ∗: p < 0.05, N versus N + SCLE, ∗∗: p < 0.01, H versus H + SCLE. L: Low glucose levels (1 mM) N: Normal glucose levels (5 mM) H: High glucose levels (25 mM). |
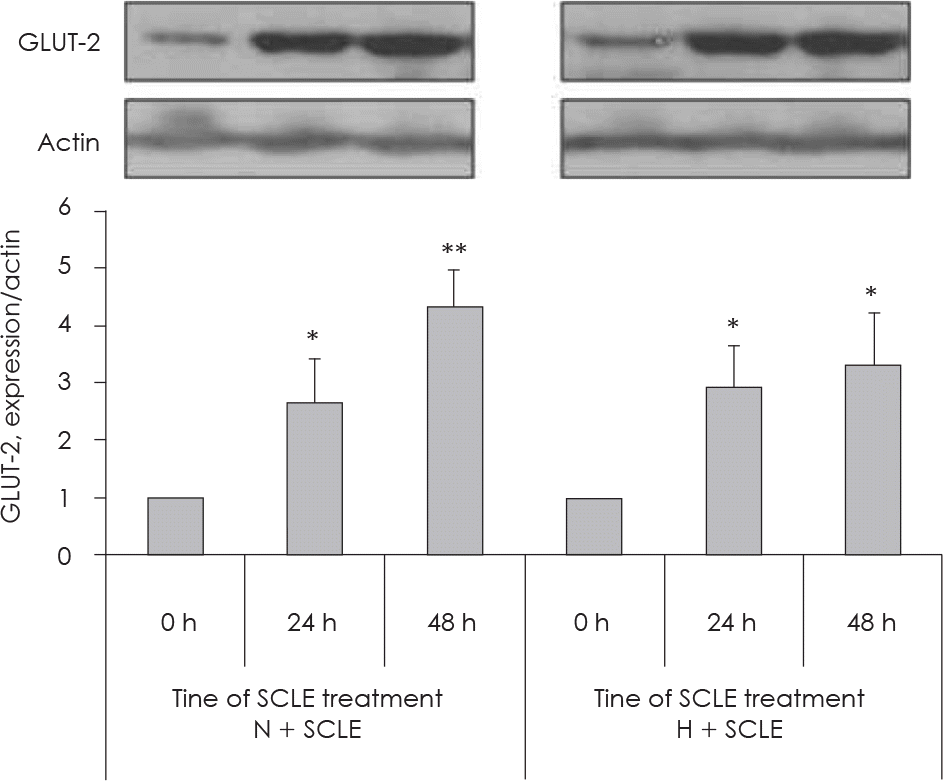 | Fig. 4.Measurement of GLUT-2 protein expression. Time-dependent effects (at 0 h, 24 h, and 48 h) of treatment with a Smilax china L. leaf extract (SCLE) on GLUT-2 expression in HepG2 cells cultured with 5 mM or 25 mM glucose. Values are the Mean ± SD of triplicate determinations. ∗: p < 0.05, ∗∗: p < 0.01 compared with 0 h N: Normal glucose levels (5 mM) H: High glucose levels (25 mM). |
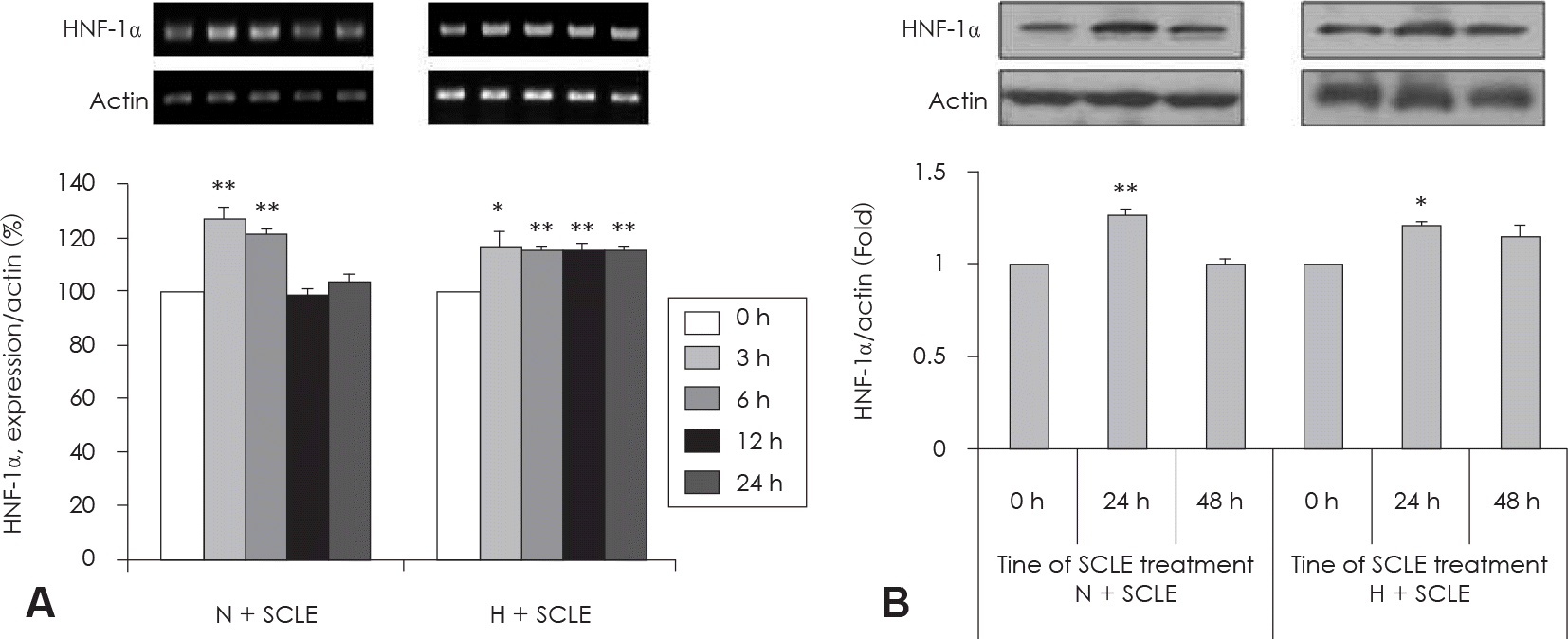 | Fig. 5.Measurement of HNF-1α mRNA and protein expression. A: Effect of a Smilax china L. leaf extract (SCLE) on HNF-1α mRNA expression in HepG2 cells. B: Effect of SCLE treatment on HNF-1α protein expression in HepG2 cells. Values are Mean ± SD of triplicate determinations. ∗: p < 0.05, N versus N + SCLE, ∗∗: p < 0.01, H versus H + SCLE N: Normal glucose levels (5 mM) H: High glucose levels (25 mM). |
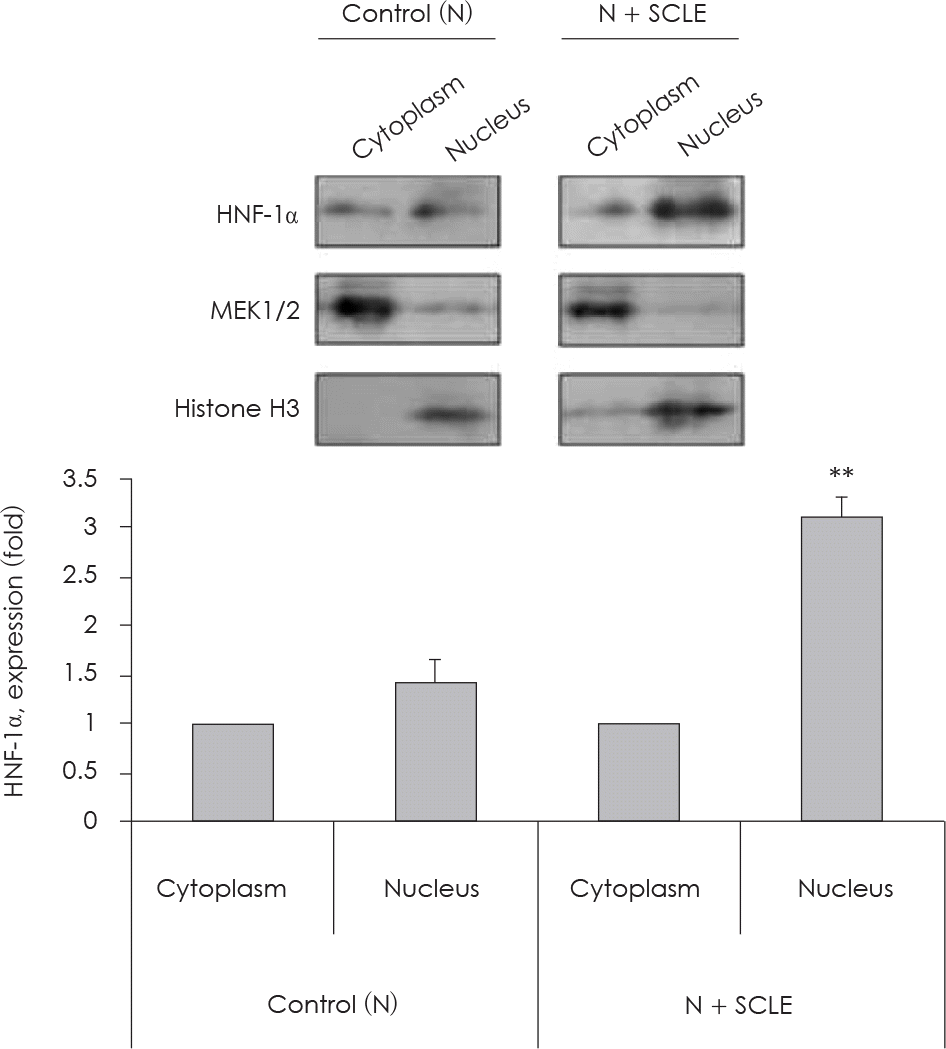 | Fig. 6.Measurement of HNF-1α expression in nucleus fraction. Effect of Smilax china L. leaf extract (SCLE) on HNF-1α protein expression in HepG2 cell fractions. Values are Mean ± SD of triplicate determination. ∗∗: p < 0.01 comparing cytoplasm and nucleus. N: Normal glucose level (5 mM). |
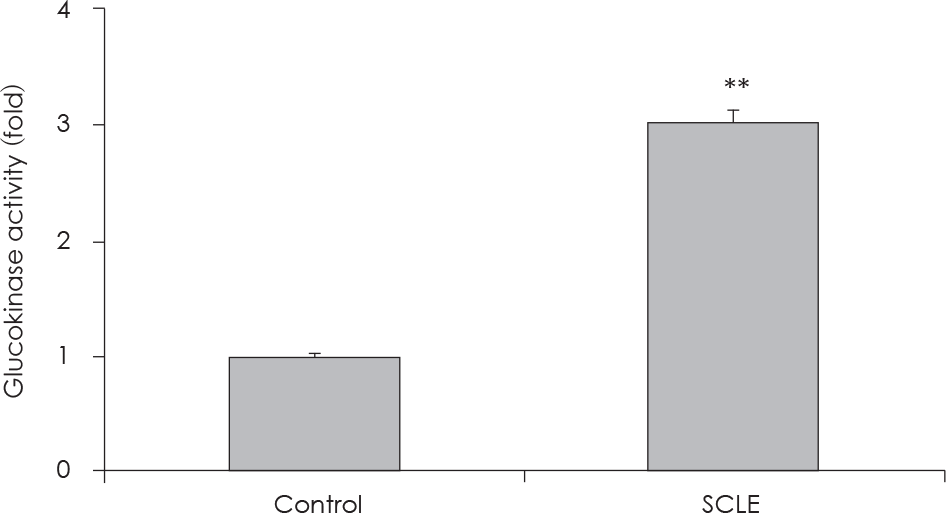 | Fig. 7.
In vitro assay to evaluate glucokinase activity. Effect of treatment with a Smilax china L. leaf extract (SCLE) on glucokinase (GK) from Bacillus stearothermophilus. Values are the Mean ± SD of triplicate determinations. ∗∗: p < 0.01, control versus SCLE (0.25 mg/ml). |
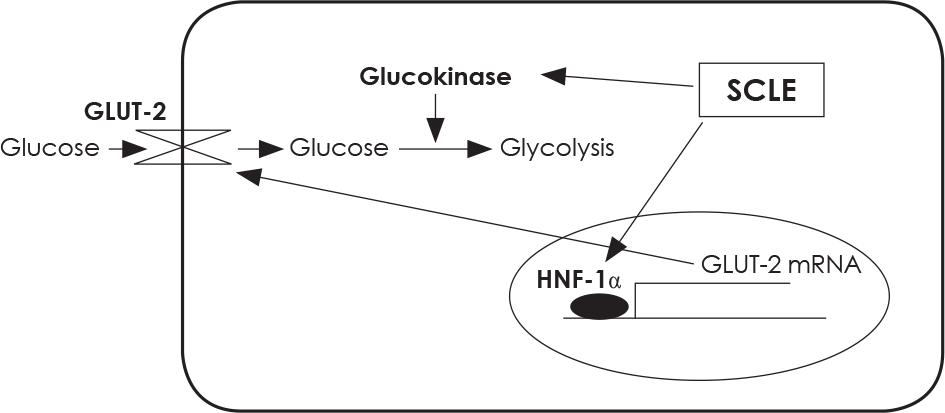 | Fig. 8.Treatment with SCLE stimulates glucose absorption and utilization by increasing GLUT-2 mRNA expression and GK activity in HepG2 cells. |
Table 1.
PCR primer sequences
Table 2.
PCR condition of each primer




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download