Abstract
Purpose
This study was conducted to establish the production conditions through optimization of the production process of beverages using Aspergillus oryzae CF1001, and to analyze volatile compounds and antidiabetic activity. Methods: The optimum condition was selected using the response surface methodology (RSM), through a regression analysis with the following independent variables gelatinization temperature (GT, X1), saccharogenic time (ST, X2), and dependent variable; ΔE value (y). The condition with the lowest ΔE value occurred with combined 45 min ST and 50℃ GT. The volatile compounds were analyzed quantitatively by GC-MS. Results: Assessment of antidiabetic activity of saccharogenic mixed grain beverage (SMGB) was determined by measurement of α-glucosidase inhibition activity, and glucose uptake activity and glucose metabolic protein expression by reverse transcriptase polymerase chain reaction (RT-PCR) and western blot analysis. Results of volatile compounds analysis, 62 kinds of volatile compounds were detected in SMGB. Palmitic acid (9.534% ratio), benzaldehyde (8.948% ratio), benzyl ethyl ether (8.792% ratio), ethyl alcohol (8.35% ratio), and 2-amyl furan (4.826% ratio) were abundant in SMGB. We confirmed that α-glucosidase inhibition activity, glucose uptake activity, and glucose-metabolic proteins were upregulated by SMGB treatment with concentration dependent manner. Conclusion: Saccharogenic mixed grain beverage (SMGB) showed potential antidiabetic activity. Further studies will be needed in order to improve the taste and functionality of SMGB.
References
1. Lee SE, Seong NS, Bang JK, Park CG, Sung JS, Song J. Antioxidative activities of Korean medicinal plants. Korean J Med Crop Sci. 2003; 11(2):127–134.
2. Kang JR, Lee SJ, Kwon HJ, Kwon MH, Sung NJ. Establishment of extraction conditions for the optimization of the black garlic antioxidant activity using the response surface methodology. Korean J Food Preserv. 2012; 19(4):577–585.

3. Hwang EY, Kim DH, Kim HJ, Hwang JY, Park TS, Lee IS, Son JH. Antioxidant activities and nitric oxide production of medicine plants in Gyeongsangbukdo (Carthamus tinctorius seed, Cyperus rotundus, Schizonepeta tenuifolia, Polygonatum odoratum var. pluriflorum, Paeonia lactiflora). J Korean Soc Appl Biol Chem. 2011; 54(3):171–177.

4. Kim DI, Hong JH. Optimization of ethanol extraction conditions for functional components from Lespedeza cuneata using response surface methodology. Korean J Food Cookery Sci. 2012; 28(3):275–283.

5. Jeong JE, Shim SP, Jeong YS, Jung HK, Kim YC, Hong JH. Optimization of extraction conditions for ethanol extracts from Citrus unshiu peel by response surface methodology. Korean J Food Preserv. 2011; 18(5):755–763.

6. Joo N, Kim B, Kim AJ. The Optimization of jelly with blueberry juice using response surface methodology. Korean J Food Nutr. 2012; 25(1):17–25.

7. Jung KI, Cho EK. Adding germinated brown rice soaked in a mycelial culture broth of Phellinus linteus to muffins: an assessment using the response surface methodology. J Korean Soc Food Sci Nutr. 2011; 40(6):892–902.

8. Nepom GT. A unified hypothesis for the complex genetics of HLA associations with IDDM. Diabetes. 1990; 39(10):1153–1157.

9. Kim DJ, Kim JM, Kim TH, Baek JM, Kim HS, Choe M. Effects of mixed extract from Lycium chinense, Cordyceps militaris and Acantopanax senticosus on glucose-regulationg enzymes of HepG2 in hyperglycemic conditions. J Korean Soc Food Sci Nutr. 2010; 39(9):1257–1262.
10. Kim HS, Kim TW, Kim DJ, Kim KK, Choe M. Effects of medicinal plant water extracts on expression of anti-diabetic enzymes mRNA. J Korean Soc Food Sci Nutr. 2013; 42(7):1008–1014.

11. Kim HS, Kim TW, Kim DJ, Lee JS, Choe M. Effects of medicinal herb water extracts on expression of hepatic glucokinase, pyruvate dehydrogenase and acetyl-CoA carboxylase mRNA. Korean J Nutr. 2013; 46(2):119–125.

12. Zhang YL, Tan XH, Xiao MF, Li H, Mao YQ, Yang X, Tan HR. Establishment of liver specific glucokinase gene knockout mice: a new animal model for screening anti-diabetic drugs. Acta Pharmacol Sin. 2004; 25(12):1659–1665.
13. Moore MC, Coate KC, Winnick JJ, An Z, Cherrington AD. Regulation of hepatic glucose uptake and storage in vivo. Adv Nutr. 2012; 3(3):286–294.

14. Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes. 1999; 48(5):1198–1214.

15. Kim DJ, Kim JM, Kim TH, Baek JM, Kim HS, Choe M. Anti-diabetic effects of mixed extracts from Lycium chinense, Cordyceps militaris, and Acanthopanax senticosus. Korean J Plant Resour. 2010; 23(5):423–429.
16. Nordlie RC, Foster JD, Lange AJ. Regulation of glucose production by the liver. Annu Rev Nutr. 1999; 19:379–406.

17. Jang EH, Ko JH, Ahn CW, Lee HH, Shin JK, Chang SJ, Park CS, Kang JH. In vivo and in vitro application of black soybean peptides in the amelioration of endoplasmic reticulum stress and improvement of insulin resistance. Life Sci. 2010; 86(7–8):267–274.

18. Lee JS, Kang YH, Kim KK, Lim JG, Kim TW, Choe M. Characteristics and antioxidative activity of fermented mixed grain beverages produced by different microbial species. J Korean Soc Food Sci Nutr. 2013; 42(8):1175–1182.

19. Hwang SH, Choi SJ, Hwang YS, Lim SS. Comparison analysis of essential oils composition in difference parts from Lindera ob-tusiloba BL. according to the season by gas Chromatography-Mass Spectrometry (GC-MS). Korean J Pharmacogn. 2013; 44(1):30–40.
20. Kim HS, Kim TW, Kim DJ, Lee JS, Kim KK, Choe M. Antioxidant activities and α-glucosidase inhibitory effect of water extracts from medicinal plants. Korean J Med Crop Sci. 2013; 21(3):197–203.

21. Choi GW, Han M, Kim Y. Development of glucoamylase & simultaneous saccharification and fermentation process for high-yield bioethanol. Korean J Biotechnol Bioeng. 2008; 23(6):499–503.
22. Parliment TH. Solvent extraction and distillation techniques. Marsili R, editor. Techniques for Analyzing Food Aroma. New York (NY): Marcel Dekker, Inc.;1997. p. 1–26.

23. Wells MJ. Chapter 2: principles of extraction and the extraction of semivolatile organics from liquids. 2.1. Principles of extraction. Somenath M, editor. Sample Preparation Techniques in Analytical Chemistry. Hoboken (NJ): John Wiley & Sons, Inc;2003. p. 37–43.
24. Cho MG, Kim H, Chae YA. Analysis of volatile compounds in leaves and fruits of Zanthoxylum schinifolium Siebold et Zucc. & Zanthoxylum piperitum DC. by headspace SPME. Korean J Med Crop Sci. 2003; 11(1):40–45.
25. Lee TS, Han EH. Volatile flavor components in mash of Takju prepared by using Aspergillus oryzae Nuruks. Korean J Food Sci Technol. 2001; 33(3):366–372.
26. Oh HS, Kim JH, Choi MY. The volatile flavor components of fresh Codonopsis lanceolata cultivated on a wild hill. Korean J Food Cookery Sci. 2006; 22(6):774–782.
27. Choi YH, Kim KH, Kang MY. Varietal difference in processing and sensory characteristics of “Sikhe” in rice. Korean J Breed. 2001; 33(2):65–72.
28. Cordero-Herrera I, Martín MÁ, Goya L, Ramos S. Cocoa flavonoids attenuate high glucose-induced insulin signalling blockade and modulate glucose uptake and production in human HepG2 cells. Food Chem Toxicol. 2014; 64:10–19.

29. Nakamaru K, Matsumoto K, Taguchi T, Suefuji M, Murata Y, Igata M, Kawashima J, Kondo T, Motoshima H, Tsuruzoe K, Miyamura N, Toyonaga T, Araki E. AICAR, an activator of AMP-activated protein kinase, downregulates the insulin receptor expression in HepG2 cells. Biochem Biophys Res Commun. 2005; 328(2):449–454.

30. Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013; 34(2–3):121–138.

31. McGowan KM, Long SD, Pekala PH. Glucose transporter gene expression: regulation of transcription and mRNA stability. Pharmacol Ther. 1995; 66(3):465–505.

32. Jung CY, Lee W. Glucose transporters and insulin action: some insights into diabetes management. Arch Pharm Res. 1999; 22(4):329–334.

33. Jung UJ, Lee MK, Park YB, Kang MA, Choi MS. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int J Biochem Cell Biol. 2006; 38(7):1134–1145.

34. Park SA, Choi MS, Kim MJ, Jung UJ, Kim HJ, Park KK, Noh HJ, Park HM, Park YB, Lee JS, Lee MK. Hypoglycemic and hypolipidemic action of Du-zhong (Eucommia ulmoides Oliver) leaves water extract in C57BL/KsJ-db/db mice. J Ethnopharmacol. 2006; 107(3):412–417.

35. Matschinsky FM. Glucokinase as glucose sensor and metabolic signal generator in pancreatic β-cells and hepatocytes. Diabetes. 1990; 39(6):647–652.

36. Shimizu T, Parker JC, Najafi H, Matschinsky FM. Control of glucose metabolism in pancreatic β-cells by glucokinase, hexokinase, and phosphofructokinase. Model study with cell lines derived from β-cells. Diabetes. 1988; 37(11):1524–1530.

37. Kim HS, Ro YJ, Choe M. Effects of Cordyceps militaris on key enzymes of carbohydrate metabolism. J Korean Soc Food Sci Nutr. 2005; 34(10):1531–1535.
38. Kim HS, Kim DJ, Hwang HJ, Lee HJ, Choe M. Hypoglycemic effect of nutraceuticals extract supplementations on NIDDM patients. J Korean Soc Appl Biol Chem. 2007; 50(1):68–71.
39. Roman-Lopez CR, Allred JB. Acute alloxan diabetes alters the activity but not the total quantity of acetyl CoA carboxylase in rat liver. J Nutr. 1987; 117(11):1976–1981.

40. Park MJ, Kang SJ, Kim AJ. Hypoglycemic effect of Angelica gigas naki extract in streptozotocin-induced diabetic rats. Korean J Food Nutr. 2009; 22(2):246–251.
Fig. 1.
Response surface plot of Brix° on the SMGB as functions of gelanization temperature and saccharogenic times.
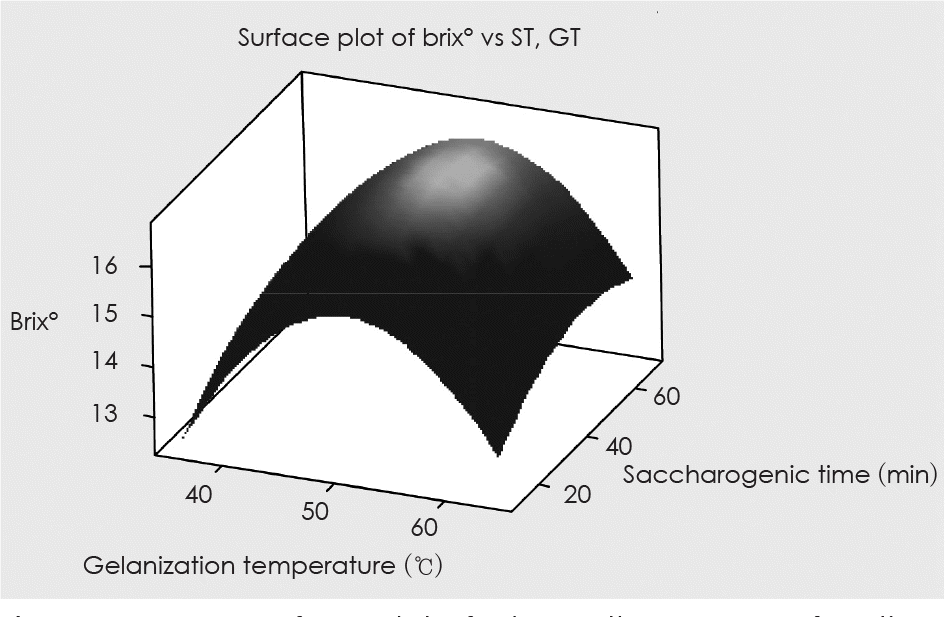
Fig. 2.
Contour map of optimzied conditions for the brix° of SMGB as functions of gelanization temperature and saccharogenic
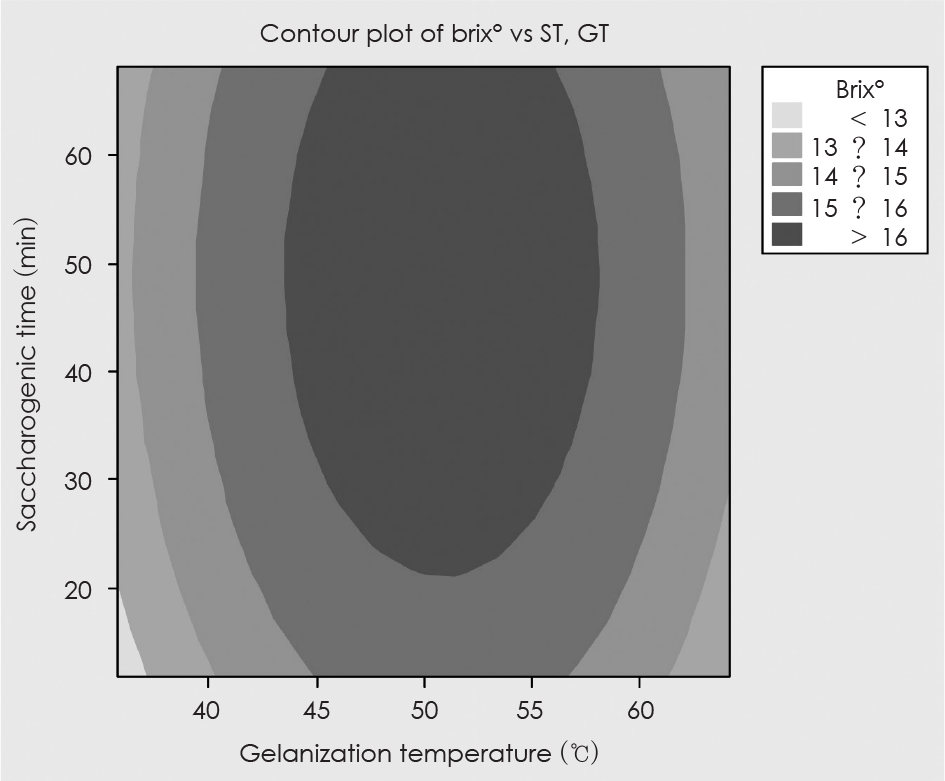
Fig. 3.
Effect of SMGB on α-glucosidase inhibition activity. Values are mean ± standard deviation of triplicate determination, different letters on the bars (a-c) indicate significant differences (p < 0.05) by Duncan's multiple range test. Acarbose Asp. CF 1001
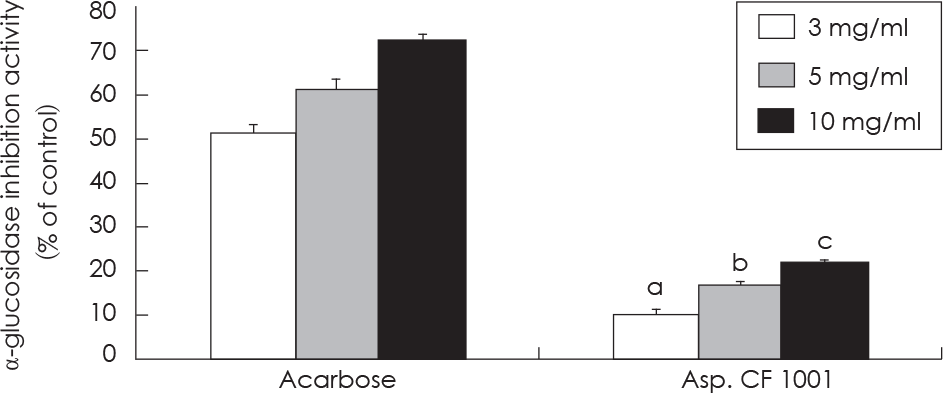
Fig. 4.
Measurement of glucose uptake activity and GLUT-2, −4 expression in HepG2 cell. A: Effect of SMGB (Asp. CF1001) on glucose uptake. B: Effect of SMGB on GLUT-2, GLUT-4 mRNA expression. C: Effect of SMGB on GLUT-2, GLUT-4 protein expression. Values are mean ± standard deviation of triplicate determination, different letters on the bars (a-d) indicate significant differences (p < 0.05) by Duncan's multiple range test. GLUT: Glucose-transporter.
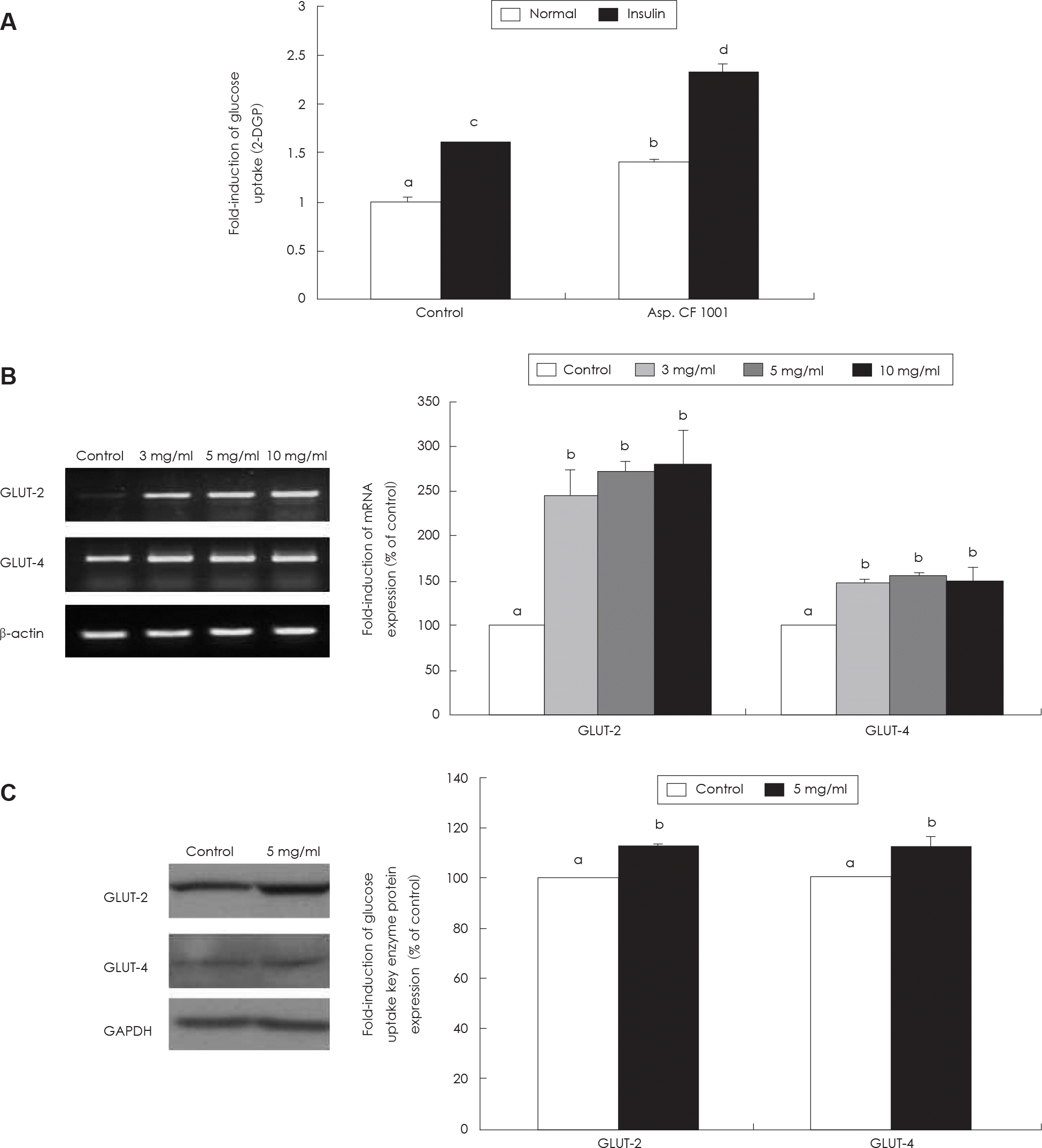
Fig. 5.
Measurement of glycolytic key enzyme expression in HepG2 cell. A: Effect of SMGB on GK and PDH mRNA expression. B: Effect of SMGB on GK and PDH protein expression. Values are mean ± standard deviation of triplicate determination, different letters on the bars (a-c) indicate significant differences (p < 0.05) by Duncan's multiple range test. GK: glucokinase, PDH: pyruvate dehydrogenase.
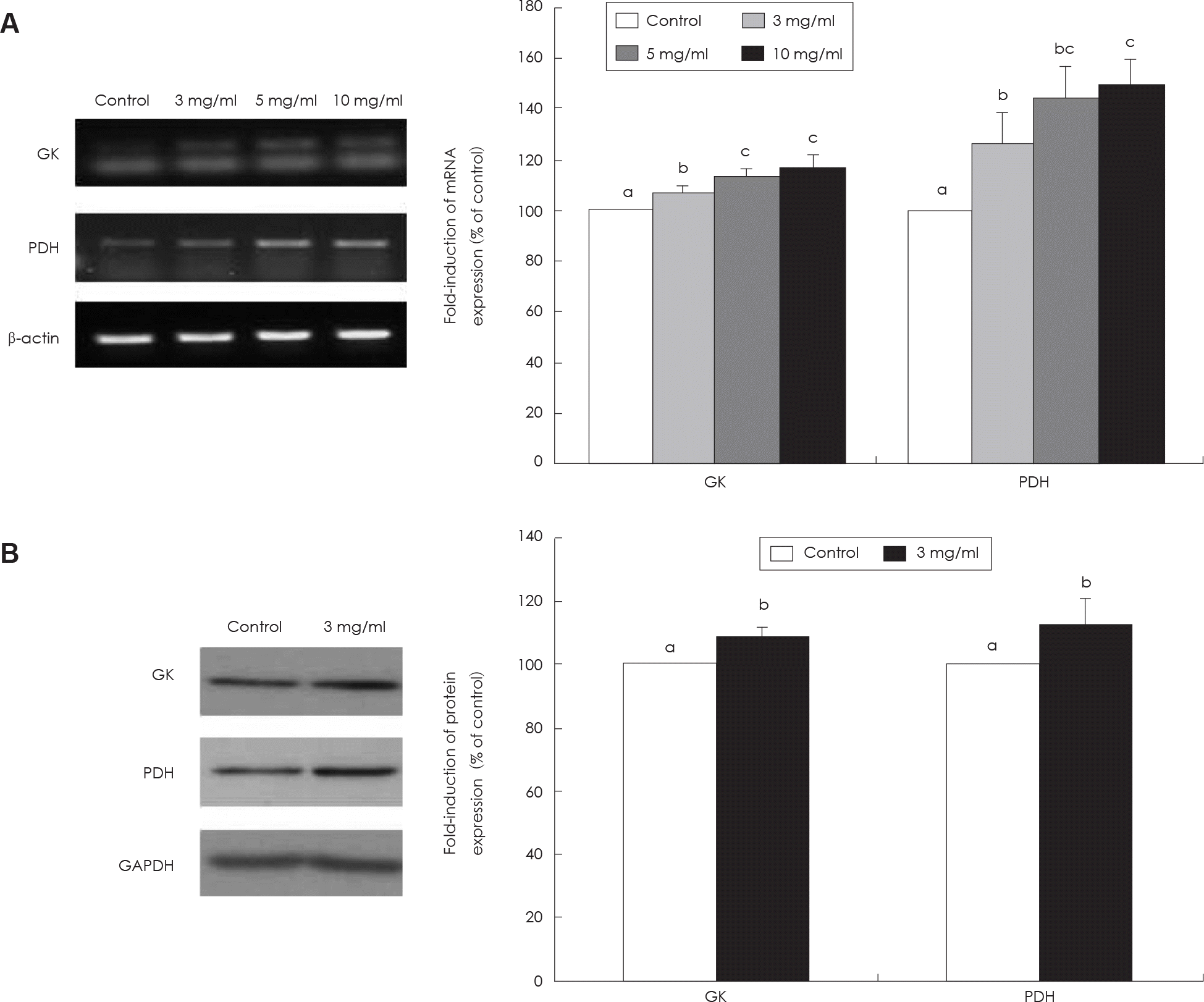
Fig. 6.
Effect of SMGB on ACL and ACC mRNA expression in HepG2 cell. Values are mean ± standard deviation of triplicate determination, different letters on the bars (a-c) indicate significant differences (p < 0.05) by Duncan's multiple range test. ACL: ATP-citrate lyase, ACC: Acetyl-CoA carboxylase.
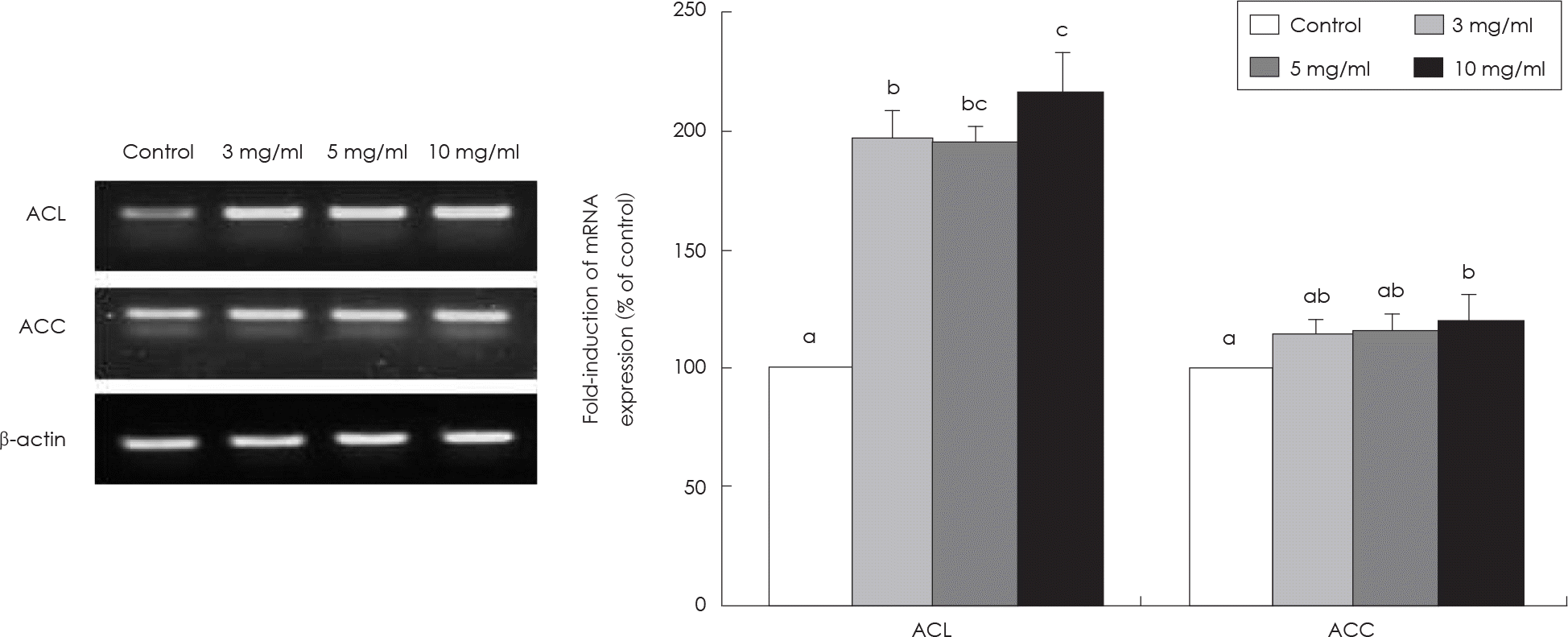
Table 1.
Central composite design for brix˚ on sacchrogeni mixed grain beverage (SMGB) with gelatinzation temperatur and saccharogenic time
| Sample | X1 | X2 |
|---|---|---|
| 01 | 50 | 40 |
| 02 | 50 | 40 |
| 03 | 50 | 40 |
| 04 | 60 | 20 |
| 05 | 64.1 | 40 |
| 06 | 50 | 40 |
| 07 | 50 | 68.3 |
| 08 | 50 | 40 |
| 09 | 35.9 | 40 |
| 10 | 50 | 11.7 |
| 11 | 60 | 60 |
| 12 | 40 | 20 |
| 13 | 40 | 60 |
Table 2.
PCR primer sequences
Table 3.
PCR condition of each primer
Table 4.
Central composite design for brix˚ on SMGB with gelat inzation temperature and saccharogenic time
Table 5.
Polynomial equation calculated for Brix° by RSM program for SMGB
| Responses | Polynomial equation | R2 | p-value |
|---|---|---|---|
| Brix° | Y = 1 6.62 + 0.2186 X1 + 0.3216 X2-1.3350 X1 2-0.36 X2 2-0.250 X1X2 | 0.9646 | 0.001 |
Table 6.
Predicted levels of optimum conditions for the maximized and minimized responses of variables by the ridge analysis of their response surface
| Response | Saccharogenic condition | |||
|---|---|---|---|---|
| X1 | X2 | Estimated responses (Max) | Morphology | |
| Brix° | 50.71 | 45.12 | 16.6876 | Maximum point |
Table 7.
Regression analysis for regression model of Brix° in sac charogenic condition of SMGB
| Extract condition | F-ratio |
|---|---|
| X1 | 194.13 |
| X2 | 14.12 |
Table 8.
Volatile compouds of SMGB




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download