Abstract
Monocyte chemoattractant protein-1 (MCP-1) plays an important role in cardiovascular disease (CVD). Genetic polymorphism in the regulatory regions of MCP-1 could affect MCP-1 expression. The purpose of the study was to explore the possible association of MCP-1 −2518 A/G genetic polymorphism and CVD risk factors in the elderly Korean population. Dietary, anthropometric, and biochemical factors were assessed in 168 subjects. The frequency of A/A, G/ A, and G/G genotypes was 14.2%, 45.8%, and 40.0%, respectively. The blood level of MCP-1 was significantly higher in subjects with A/A genotype. The MCP-1 level was significantly higher in A/A genotype with hypercholesterolemia than in other genotypes. Meat intake and percent energy from lipids were significantly positively correlated with the MCP-1 level, especially, stronger in A/A genotype. In the stepwise discriminant analysis, TNF-α level, meat intake, HDL-C were associated with MCP-1 in all subjects (model R2 = 24%). TNF-α level, sugar intake, cholesterol intake, and meat intake affected MCP-1 in A/A genotype (model R2 = 82%), but not in G/A or G/G. In conclusion, subjects pos-sessing A/A genotype exhibited higher levels of MCP-1 than other genotypes in Korean elders. Further, meat, sugar, and cholesterol intakes affected the MCP-1 level. Therefore, the decrement of meat, sugar, and cholesterol intakes helps to normalize the MCP-1 level and can decrease CVD risk in A/A genotype. (J Nutr Health 2013; 46(6): 511 – 520)
Go to : 
References
1). Statistics Korea. Deaths and death rates. The Cause of Death Statistics 2011. Seoul: Statistics Korea;2012. p. 3.
2). Koh KK, Han SH, Quon MJ. Inflammatory markers and the metabolic syndrome: insights from therapeutic interventions. J Am Coll Cardiol. 2005; 46(11):1978–1985.
4). Melgarejo E, Medina MA, Sánchez-Jiménez F, Urdiales JL. Monocyte chemoattractant protein-1: a key mediator in inflammatory processes. Int J Biochem Cell Biol. 2009; 41(5):998–1001.

5). Ylä-Herttuala S, Lipton BA, Rosenfeld ME, Särkioja T, Yoshimura T, Leonard EJ, Witztum JL, Steinberg D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 1991; 88(12):5252–5256.
6). Nelken NA, Coughlin SR, Gordon D, Wilcox JN. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991; 88(4):1121–1127.

7). Seli E, Pehlivan T, Selam B, Garcia-Velasco JA, Arici A. Estradiol downregulates MCP-1 expression in human coronary artery endothelial cells. Fertil Steril. 2002; 77(3):542–547.

8). de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, McCabe CH, Cannon CP, Braunwald E. Association between plasma levels of monocyte chemoattractant protein-1 and longterm clinical outcomes in patients with acute coronary syndromes. Circulation. 2003; 107(5):690–695.

9). Serrano-Martínez M, Palacios M, Lezaun R. Monocyte chemoattractant protein-1 concentration in coronary sinus blood and severity of coronary disease. Circulation. 2003; 108(10):e75.

10). Cipollone F, Marini M, Fazia M, Pini B, Iezzi A, Reale M, Palos-cia L, Materazzo G, D'Annunzio E, Conti P, Chiarelli F, Cuccu-rullo F, Mezzetti A. Elevated circulating levels of monocyte chemoattractant protein-1 in patients with restenosis after coronary angioplasty. Arterioscler Thromb Vasc Biol. 2001; 21(3):327–334.

11). Deo R, Khera A, McGuire DK, Murphy SA, Meo Neto Jde P, Morrow DA, de Lemos JA. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Am Coll Cardiol. 2004; 44(9):1812–1818.

12). Rovin BH, Lu L, Saxena R. A novel polymorphism in the MCP-1 gene regulatory region that influences MCP-1 expression. Biochem Biophys Res Commun. 1999; 259(2):344–348.

13). Kroner A, Mäurer M, Loserth S, Kleinschnitz C, Hemmer B, Ros-che B, Toyka KV, Rieckmann P. Analysis of the monocyte chemoattractant protein 1–2518 promoter polymorphism in patients with multiple sclerosis. Tissue Antigens. 2004; 64(1):70–73.
14). Krüger B, Schröppel B, Ashkan R, Marder B, Zülke C, Murphy B, Krämer BK, Fischereder M. A monocyte chemoattractant protein-1 (MCP-1) polymorphism and outcome after renal transplantation. J Am Soc Nephrol. 2002; 13(10):2585–2589.

15). Szalai C, Duba J, Prohászka Z, Kalina A, Szabó T, Nagy B, Hor-váth L, Császár A. Involvement of polymorphisms in the chemokine system in the susceptibility for coronary artery disease (CAD). Coincidence of elevated Lp(a) and MCP-1–2518 G/G genotype in CAD patients. Atherosclerosis. 2001; 158(1):233–239.
16). Tabara Y, Kohara K, Yamamoto Y, Igase M, Nakura J, Kondo I, Miki T. Polymorphism of the monocyte chemoattractant protein (MCP-1) gene is associated with the plasma level of MCP-1 but not with carotid intima-media thickness. Hypertens Res. 2003; 26(9):677–683.

17). Buraczynska M, Bednarek-Skublewska A, Buraczynska K, Ksi-azek A. Monocyte chemoattractant protein-1 (MCP-1) gene polymorphism as a potential risk factor for cardiovascular disease in hemodialyzed patients. Cytokine. 2008; 44(3):361–365.

18). Jeon HJ, Choi HJ, Park BH, Lee YH, Oh T. Association of monocyte chemoattractant protein-1 (MCP-1) 2518A/G polymorphism with proliferative diabetic retinopathy in Korean type 2 diabetes. Yonsei Med J. 2013; 54(3):621–625.

19). Moon JY, Jeong L, Lee S, Jeong K, Lee T, Ihm CG, Suh J, Kim J, Jung YY, Chung JH. Association of polymorphisms in monocyte chemoattractant protein-1 promoter with diabetic kidney failure in Korean patients with type 2 diabetes mellitus. J Korean Med Sci. 2007; 22(5):810–814.

20). Stang J, Zephier EM, Story M, Himes JH, Yeh JL, Welty T, Howard BV. Dietary intakes of nutrients thought to modify cardiovascular risk from three groups of American Indians: the Strong Heart Dietary Study, Phase II. J Am Diet Assoc. 2005; 105(12):1895–1903.

21). Tell GS, Evans GW, Folsom AR, Shimakawa T, Carpenter MA, Heiss G. Dietary fat intake and carotid artery wall thickness: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 1994; 139(10):979–989.

22). de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mame-lle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999; 99(6):779–785.
23). Kreijkamp-Kaspers S, Kok L, Bots ML, Grobbee DE, Lampe JW, van der Schouw YT. Randomized controlled trial of the effects of soy protein containing isoflavones on vascular function in postmenopausal women. Am J Clin Nutr. 2005; 81(1):189–195.

24). Jiang R, Jacobs DR Jr, Mayer-Davis E, Szklo M, Herrington D, Jenny NS, Kronmal R, Barr RG. Nut and seed consumption and inflammatory markers in the multiethnic study of atherosclerosis. Am J Epidemiol. 2006; 163(3):222–231.

25). Park HJ. Association of MCP-1 polymorphism with cardiovascular risk factors in Korean elderly [Ph.D. thesis]. Seoul: Ewha Womans University;2007.
26). The Korean Nutrition Society, Korean Nutrition Information Center. Nutritional assessment program, ‘CAN pro 3.0' [CD-ROM]. Seoul: The Korean Nutrition Society;2006.
27). The Korean Nutrition Society. Dietary reference intakes for Koreans. Seoul: The Korean Nutrition Society;2010.
28). Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18(6):499–502.

29). Kitamoto S, Egashira K. Anti-monocyte chemoattractant protein-1 gene therapy for cardiovascular diseases. Expert Rev Cardiovasc Ther. 2003; 1(3):393–400.

30). Okopień B, Haberka M, Cwalina L, Kowalski J, Belowski D, Madej A, Zieliński M, Krysiak R, Labuzek K, Kalina Z, Herman ZS. Plasma cytokines as predictors of coronary heart disease. Res Commun Mol Pathol Pharmacol. 2002; 112(1–4):5–15.
31). Ozyürek AR, Gürses D, Ulger Z, Levent E, Bakiler AR, Berdeli A. Allelic frequency of the MCP-1 promoter-2518 polymorphism in the Turkish population and in Turkish patients with juvenile rheumatoid arthritis. Clin Rheumatol. 2007; 26(4):546–550.
32). Kim HL, Yang SH, Oh YK, Lee JE, Oh JE, Yoon HJ, Kim YS, Ahn CR, Han JS, Kim SG, Lee JS. The effects of polymorphism in the MCP-1 gene regulatory region on MCP-1 expression and the manifestation of lupus nephritis. Korean J Nephrol. 2002; 21(1):137–144.
33). Pae CU, Kim JJ, Yu HS, Lee CU, Lee SJ, Jun TY, Lee C, Paik IH. Monocyte chemoattractant protein-1 promoter-2518 polymorphism may have an influence on clinical heterogeneity of bipolar I disorder in the Korean population. Neuropsychobiology. 2004; 49(3):111–114.
34). Zhong C, Luzhan Z, Genshan M, Jiahong W, Xiaoli Z, Qi Q. Monocyte chemoattractant protein-1–2518 G/A polymorphism, plasma levels, and premature stable coronary artery disease. Mol Biol Rep. 2010; 37(1):7–12.

35). Pola R, Flex A, Gaetani E, Proia AS, Papaleo P, Di Giorgio A, Straface G, Pecorini G, Serricchio M, Pola P. Monocyte chemoattractant protein-1 (MCP-1) gene polymorphism and risk of Alzheimer's disease in Italians. Exp Gerontol. 2004; 39(8):1249–1252.

36). Zietz B, Büchler C, Herfarth H, Müller-Ladner U, Spiegel D, Schölmerich J, Schäffler A. Caucasian patients with type 2 diabetes mellitus have elevated levels of monocyte chemoattractant protein-1 that are not influenced by the −2518 A–>G promoter polymorphism. Diabetes Obes Metab. 2005; 7(5):570–578.
37). Aguilar F, González-Escribano MF, Sánchez-Román J, Núñez-Roldán A. MCP-1 promoter polymorphism in Spanish patients with systemic lupus erythematosus. Tissue Antigens. 2001; 58(5):335–338.

38). Penz P, Bucova M, Lietava J, Blazicek P, Paulovicova E, Mrazek F, Bernadic M, Buckingham TA, Petrek M. MCP-1–2518 A/G gene polymorphism is associated with blood pressure in ischemic heart disease asymptomatic subjects. Bratisl Lek Listy. 2010; 111(8):420–425.
39). Sonnenberg L, Pencina M, Kimokoti R, Quatromoni P, Nam BH, D'Agostino R, Meigs JB, Ordovas J, Cobain M, Millen B. Dietary patterns and the metabolic syndrome in obese and non-obese Framingham women. Obes Res. 2005; 13(1):153–162.

40). Franz MJ, Bantle JP, Beebe CA, Brunzell JD, Chiasson JL, Garg A, Holzmeister LA, Hoogwerf B, Mayer-Davis E, Mooradian AD, Purnell JQ, Wheeler M. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2002; 25(1):148–198.

41). Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997; 337(21):1491–1499.

42). Shishehbor F, Roche HM, Gibney MJ. The effect of low and moderate fat intakes on the postprandial lipaemic and hormonal responses in healthy volunteers. Br J Nutr. 1999; 81(1):25–30.

Go to : 
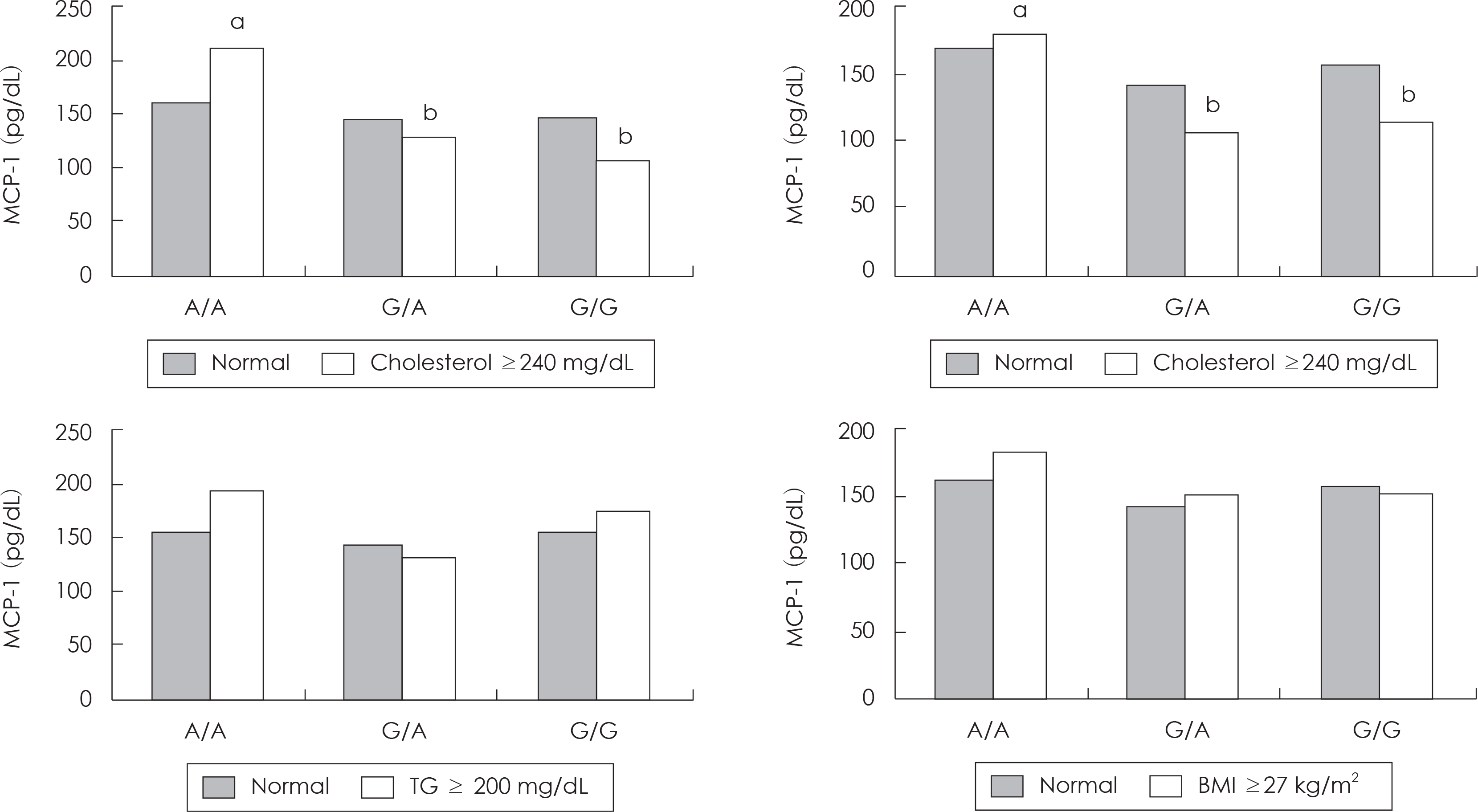 | Fig. 1.The level of MCP-1 according to genotype in the subject with dyslipideamia and obese. ab: Different superscript letters indicate the comparison with significant differences according to MCP-1 genotypes within the same category by GLM test at p < 0.05. |
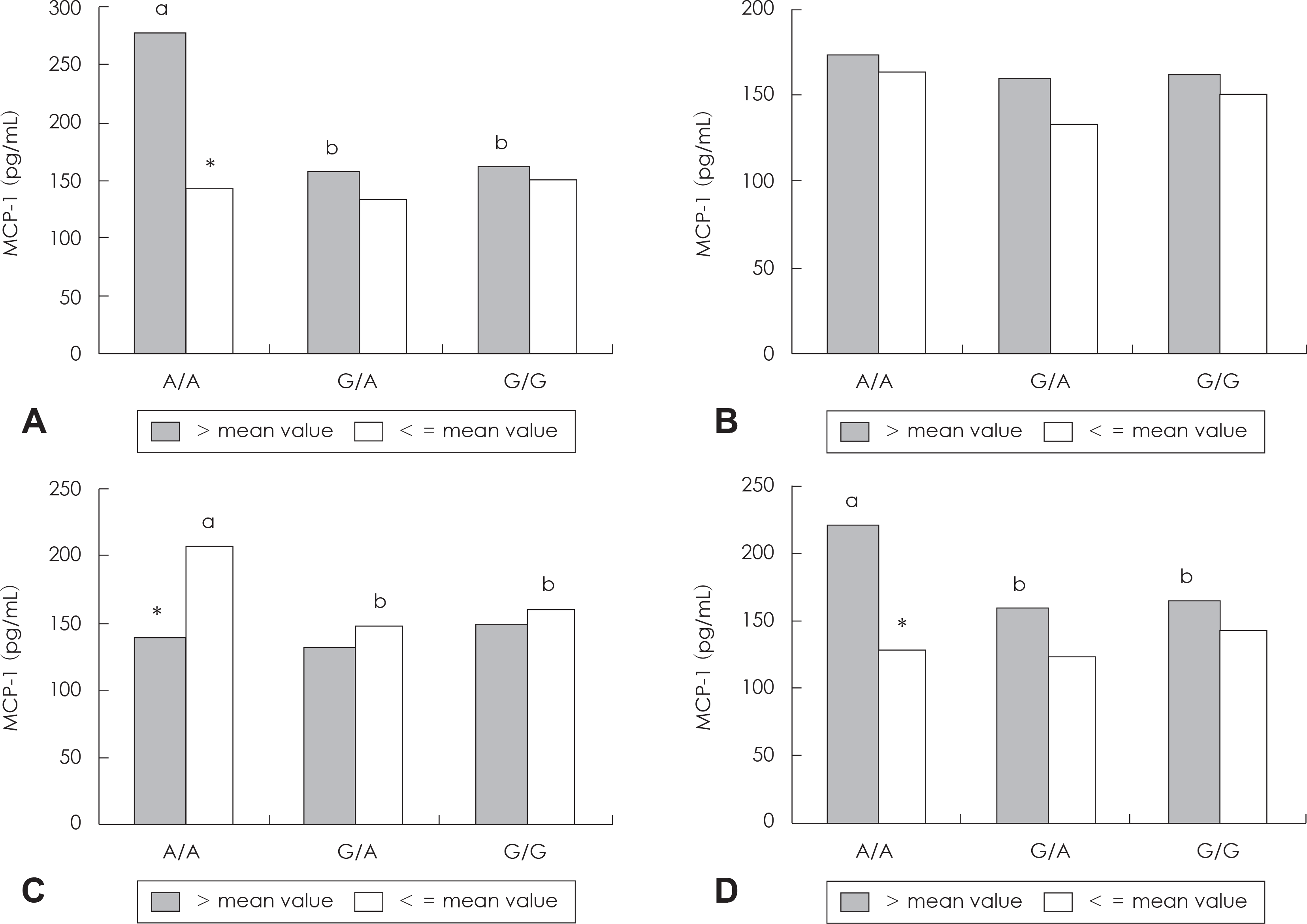 | Fig. 2.The plasma MCP-1 level according to intake of meat (A), sugar (B), % energy from carbohydrate (C), % energy from lipid (D) by genotype. ab: Different superscript letters indicate the comparison with significant differences according to MCP-1 genotypes within the same category by GLM test at p < 0.05. *: Significant differences according to intake levels within the same genotypes by Student's t-test, p < 0.05. |
Table 1.
Clinical characteristics of subjects based on MCP-1 polymorphism
| All | A/A | G/A | G/G | |
|---|---|---|---|---|
| MCP-1 genotype [ n, (%)] | 168 (100) | 24 (14.2) | 77 (45.8) | 67 (40.0) |
| Age | 73.41) ± 0.4 | 72.6 ± 1.3 | 73.5 ± 0.7 | 73.5 ± 0.7 |
| Anthropometric parameters | ||||
| Height (cm) | 149.8 ± 0.4 | 150.1 ± 1.1 | 149.6 ± 0.7 | 150.1 ± 0.7 |
| Weight (kg) | 56.3 ± 0.7 | 55.4 ± 1.5 | 57.3 ± 1.0 | 55.5 ± 1.2 |
| BMI (kg/m 2)2) | 24.9 ± 0.2 | 24.6 ± 0.6 | 25.5 ± 0.3 | 24.5 ± 0.4 |
| Waist (cm) | 81.7 ± 0.6 | 80.1 ± 1.5 | 82.6 ± 0.9 | 81.3 ± 1.1 |
| WHR 3) | 0.85 ± 0.04 | 0.84 ± 0.01 | 0.86 ± 0.01 | 0.84 ± 0.01 |
| TSF (mm)4) | 20.9 ± 0.4 | 20.9 ± 1.2 | 21.1 ± 0.7 | 20.7 ± 0.7 |
| Body fat (%) | 32.1 ± 0.4 | 31.6 ± 1.2 | 32.7 ± 0.6 | 31.6 ± 0.7 |
| Fat free mass (kg) | 37.9 ± 0.3 | 37.6 ± 0.9 | 38.3 ± 0.6 | 37.5 ± 0.6 |
| Hematological variables | ||||
| Fasting blood glucose (mg/dL) | 109.8 ± 3.5 | 99.2 ± 4.5 | 115.2 ± 5.9 | 107.3 ± 5.4 |
| Total cholesterol (mg/dL) | 199.7 ± 2.7 | 208.1 ± 6.7 | 200.6 ± 4.4 | 195.4 ± 4.0 |
| LDL-cholesterol (mg/dL) | 124.7 ± 2.7 | 133.9 ± 7.2 | 126.9 ± 4.5 | 118.7 ± 3.6 |
| HDL-cholesterol (mg/dL) | 49.3 ± 0.9 | 48.1 ± 2.3 | 49.8 ± 1.5 | 49.1 ± 1.5 |
| Triglyceride (mg/dL) | 128.2 ± 4.0 | 130.9 ± 10.7 | 118.7 ± 5.0 | 138.7 ± 7.8 |
| Blood pressure | ||||
| SBP (mmHg)5) | 144.8 ± 1.6 | 140.4 ± 4.6 | 145.6 ± 2.2 | 145.0 ± 2.6 |
| DBP (mmHg)6) | 82.1 ± 0.9 | 81.0 ± 3.8 | 83.7 ± 1.3 | 80.8 ± 1.3 |
Table 2.
Daily food intakes by food groups based on MCP-1 polymorphism
| Food group (g) | All (n = 168) | A/A (n = 24) | G/A (n = 77) | G/G (n = 67) |
|---|---|---|---|---|
| Meats | 51.6 ± 5.71) | 41.3 ± 12.1 | 56.6 ± 8.30 | 49.7 ± 9.90 |
| Fishes | 36.5 ± 5.10 | 36.9 ± 14.5 | 48.5 ± 9.40 | 22.5 ± 4.30 |
| Eggs | 13.7 ± 2.00 | 15.3 ± 4.40 | 9.49 ± 2.20 | 17.9 ± 4.20 |
| Milk products | 102.1 ± 11.40 | 109.9 ± 32.60 | 103.7 ± 17.70 | 97.6 ± 16.7 |
| Animal total | 203.9 ± 14.60 | 203.4 ± 42.30 | 218.3 ± 21.80 | 187.7 ± 22.30 |
| Cereals | 246.1 ± 7.800 | 259.6 ± 23.70 | 229.1 ± 10.60 | 260.8 ± 12.80 |
| Potatoes | 33.7 ± 6.20 | 24.9 ± 10.0 | 28.5 ± 6.70 | 42.9 ± 13.2 |
| Vegetables | 258.9 ± 14.80 | 237.3 ± 35.60 | 293.8 ± 26.80 | 226.7 ± 16.20 |
| Fruits | 152.7 ± 18.50 | 162.6 ± 51.90 | 138.6 ± 30.10 | 165.3 ± 24.90 |
| Seaweeds | 3.51 ± 0.65 | 3.42 ± 1.47 | 3.09 ± 0.91 | 4.03 ± 1.14 |
| Mushrooms | 0.98 ± 0.32 | 1.40 ± 1.24 | 0.74 ± 0.47 | 1.11 ± 0.40 |
| Legume | 41.7 ± 4.40 | 47.1 ± 17.1 | 38.5 ± 5.70 | 43.3 ± 6.50 |
| Nuts | 6.27 ± 2.31 | 1.36 ± 1.24 | 9.39 ± 4.74 | 4.46 ± 1.88 |
| Sugar | 5.35 ± 0.81 | 8.23 ± 2.19 | 3.68 ± 0.62 | 6.23 ± 1.73 |
| Vegetable oil | 4.86 ± 0.45 | 4.82 ± 1.33 | 4.66 ± 0.54 | 5.11 ± 0.84 |
| Plant total | 754.1 ± 27.70 | 750.6 ± 85.40 | 750.1 ± 42.90 | 759.9 ± 38.80 |
| Others | 55.4 ± 5.20 | 58.8 ± 12.1 | 60.7 ± 8.50 | 48.1 ± 7.50 |
| Total | 1,013.5 ± 33.600, | 1,013.0 ± 99.900, | 1,029.1 ± 53.000, | 995.8 ± 46.80 |
Table 3.
Daily nutrient intakes based on MCP-1 polymorphism
| Nutrient | All (n = 168) | A/A (n = 24) | G/A (n = 77) | G/G (n = 67) |
|---|---|---|---|---|
| Energy (kcal) | 1,421.5 ± 36.51) | 1,417.6 ± 97.600, | 1376.4 ± 51.500 | 1474.8 ± 60.700 |
| (88.8 ± 2.1)0 | (88.6 ± 5.9)0 | (86.0 ± 2.9)0 | (92.1 ± 3.2)0 | |
| Protein (g) | 56.2 ± 1.90 | 53.5 ± 4.90 | 57.3 ± 2.90 | 55.9 ± 2.60 |
| (160.6 ± 5.6)00 | (152.7 ± 14.2)0 | (163.7 ± 8.4)00 | (159.8 ± 9.1)00 | |
| Fat (g) | 30.3 ± 1.51 | 28.6 ± 3.40 | 30.4 ± 2.20 | 30.8 ± 2.60 |
| Carbohydrate (g) | 231.2 ± 6.000 | 233.5 ± 17.10 | 218.6 ± 8.700 | 244.7 ± 9.400 |
| Fiber (g) | 5.79 ± 0.21 | 5.67 ± 0.54 | 5.89 ± 0.34 | 5.72 ± 0.27 |
| Ca (mg) | 473.1 ± 19.50 | 473.8 ± 67.10 | 491.1 ± 25.80 | 452.0 ± 30.90 |
| (83.0 ± 3.5)0 | (83.1 ± 10.8) | (86.1 ± 4.3)0 | (79.3 ± 5.1)0 | |
| P (mg) | 831.4 ± 26.90 | 835.9 ± 80.10 | 835.4 ± 39.30 | 825.2 ± 41.90 |
| (143.3 ± 4.6)00 | (144.1 ± 13.8)0 | (144.0 ± 6.7)00 | (142.3 ± 7.2)00 | |
| Fe (mg) | 11.1 ± 0.30 | 10.7 ± 0.90 | 11.1 ± 0.50 | 11.3 ± 0.50 |
| (191.4 ± 4.3)00 | (184.4 ± 11.9)0 | (191.4 ± 6.8)00 | (194.8 ± 7.0)00 | |
| Zn (mg) | 7.34 ± 0.27 | 7.51 ± 0.65 | 6.90 ± 0.26 | 7.78 ± 0.57 |
| (123.6 ± 4.6)00 | (125.9 ± 10.8)0 | (115.9 ± 4.4)00 | (131.4 ± 9.8)00 | |
| Vitamin A (μ g RE) | 539.5 ± 35.40 | 500.4 ± 81.10 | 603.3 ± 58.60 | 480.3 ± 49.40 |
| (131.6 ± 8.2)00 | (122.1 ± 18.7)0 | (147.2 ± 13.4)0 | (117.2 ± 11.5)0 | |
| Vitamin B1 (mg) | 0.87 ± 0.03 | 0.76 ± 0.06 | 0.87 ± 0.04 | 0.90 ± 0.05 |
| (96.7 ± 3.5)0 | (84.4 ± 6.9)0 | (96.7 ± 4.7)0 | (100.0 ± 6.6)00 | |
| Vitamin B2 (mg) | 0.79 ± 0.03 | 0.69 ± 0.07 | 0.82 ± 0.04 | 0.79 ± 0.05 |
| (79.1 ± 3.0)0 | (69.3 ± 7.4)0 | (81.6 ± 4.3)0 | (79.8 ± 5.1)0 | |
| Vitamin C (mg) | 84.9 ± 3.60 | 82.2 ± 12.6 | 85.5 ± 7.40 | 85.3 ± 6.40 |
| (113.3 ± 6.1)00 | (109.7 ± 16.8)0 | (113.9 ± 9.9)00 | (113.8 ± 8.6)00 | |
| Vitamin E (mg) | 7.61 ± 0.44 | 7.53 ± 1.50 | 7.48 ± 0.50 | 7.89 ± 0.80 |
| Folic acid (mg) | 231.7 ± 8.900 | 223.9 ± 24.00 | 224.4 ± 13.60 | 243.0 ± 13.60 |
| (72.4 ± 2.7)0 | (69.9 ± 7.5)0 | (70.1 ± 4.3)0 | (75.9 ± 4.3)0 | |
| Cholesterol (mg) | 169.5 ± 1.100 | 180.1 ± 30.80 | 165.0 ± 15.20 | 170.7 ± 22.20 |
| Energy distribution | ||||
| % carbohydrate | 66.0 ± 0.80 | 65.9 ± 2.10 | 64.3 ± 1.20 | 67.6 ± 1.30 |
| % protein | 15.6 ± 0.30 | ,14.8 ± 0.6 a2) | 16.5 ± 0.4 b | .14.9 ± 0.4 ab |
| % fat | 18.4 ± 0.70 | 18.1 ± 1.50 | 19.2 ± 1.00 | 17.6 ± 1.10 |
Table 4.
Immune variables based on MCP-1 polymorphism
| All (n = 168) | A/A (n = 24) | G/A (n = 77) | G/G (n = 67) | |
|---|---|---|---|---|
| MCP-1 (pg/mL) | 169.9 ± 8.91) | 177.7 ± 19.4 a2) | 143.7 ± 9.52 b | 151.3 ± 11.7 b |
| IL-2 (pg/mL) | 19.1 ± 0.3 | 19.2 ± 1.5 | 18.5 ± 0.5 | 19.5 ± 0.4 |
| IL-6 (pg/mL) | 3.27 ± 0.35 | 5.04 ± 2.23 | 3.11 ± 0.22 | 2.77 ± 0.24 |
| TNF-α (pg/mL) | 9.51 ± 0.18 | 9.71 ± 0.42 | 9.35 ± 0.25 | 9.62 ± 0.30 |
| Complement 3 (g/L) | 0.76 ± 0.01 | 0.75 ± 0.02 | 0.75 ± 0.01 | 0.77 ± 0.02 |
Table 5.
Pearson's correlation coefficients between food intakes and plasma MCP-1 level
| All (n = 168) | A/A (n = 24) | G/A (n = 77) | G/G (n = 67) | |
|---|---|---|---|---|
| Cereal | –0.136121) | –0.41217**2) | –0.12857 | –0.09008 |
| Potato | –0.07582 | –0.13981 | –0.18521 | 0.10134 |
| Sugar | –0.02572 | –0.02783 | –0.13544 | –0.15419 |
| Nut | –0.01008 | –0.00663 | –0.12863 | –0.12387 |
| Vegetable | –0.07588 | –0.00886 | –0.14774 | 0.02885 |
| Fruit | –0.05231 | –0.12482 | –0.01817 | 0.09357 |
| Meat | –0.17872** | –0.59388** | –0.14200 | 0.12988 |
| Fish | –0.12341 | –0.02694 | –0.27373** | 0.02048 |
| Seafood | –0.08521 | –0.05932 | –0.09108 | –0.05562 |
| Animal food | –0.04015 | –0.23874 | –0.07993 | 0.08818 |
| Plant food | –0.08087 | –0.02631 | –0.13091 | 0.12107 |
| Total food intake | –0.14571 | –0.16870 | –0.10703 | 0.18246 |
| Cholesterol | –0.04129 | –0.16617 | –0.02519 | 0.07313 |
| % CHO | –0.13949** | –0.42885** | –0.12580 | –0.09861 |
| % protein | –0.02698 | –0.28628 | –0.16616 | 0.13462 |
| % lipid | –0.17821** | –0.54005** | –0.17330 | 0.09218 |
Table 6.
The result of discriminant analysis for factors related the MCP-1 level




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download